You are here: vision-research.eu » Vision Research » The Young Researchers View » Christina Schwarz (Q03-2018)
The Research of Christina Schwarz
 |
Towards safe two-photon ophthalmoscopy in the living human eye
Many blinding retinal diseases cause severe alterations in visual cycle function that precede any detectable change in cellular morphology in the retina. The visual cycle is a sequence of biochemical reactions that stepwise regenerates the visual pigment and is essential to maintain human vision. To diagnose any malfunctioning and develop adequate treatment, there is a need for objective methods that can quantify visual cycle kinetics in the living eye. Two-photon ophthalmoscopy shows potential to fill this gap with the advantages of superior resolution compared to electroretinography and sparing from artifacts pigment densitometry is afflicted with.
Several years ago, two-photon ophthalmoscopy (TPO) was demonstrated in the living primate eye [1]. TPO uses an ultrashort pulse laser as imaging light source and piggybacks on the technology of custom adaptive optics scanning light ophthalmoscopes (AOSLOs) to maximize excitation efficiency while minimizing the required light levels. For my PhD Thesis research in Pablo Artal’s lab in Murcia, Spain, I was working with a binocular adaptive optics visual simulator and received extensive training in physiological and adaptive optics. As a postdoc at the University of Rochester, NY, I expanded my expertise towards retinal imaging. With the guidance of Jennifer Hunter and David Williams I have been pursuing the goal of safely translating TPO to human.
Cellular-scale functional two-photon retinal imaging
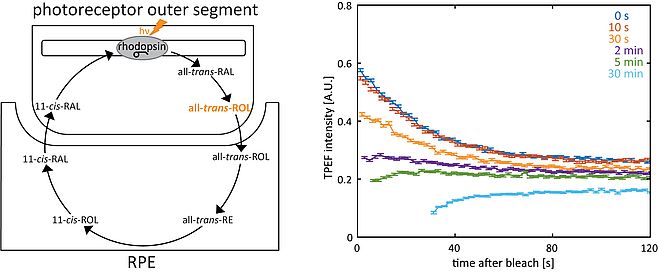
Rods and cones can routinely be imaged in the living macaque eye by capturing reflected light and two-photon excited fluorescence (TPEF) (Fig. 1). TPEF from photoreceptors shows a strongly time-dependent behavior, which is mostly due to the fluorescence of all-trans-retinol and its transient existence during the visual cycle (Fig. 2). Bleaching experiments in the living monkey eye revealed that TPEF from rods and cones showed distinct time courses in response to the imaging laser, reflecting differences in stimulation and rates of pigment recovery [2]. During dark adaptation, cone TPEF decreases about 4x faster than rod TPEF. The rates of fluorescence decrease in rods and cones in turn are approximately 4x greater than their respective photopigment regeneration rates, which indicates that the tracked fluorophore is related to an intermediate step in the visual cycle. The increase in TPEF from rods after incremental bleaches corresponds to the fraction of rhodopsin bleached [3].
Safety of functional two-photon ophthalmoscopy
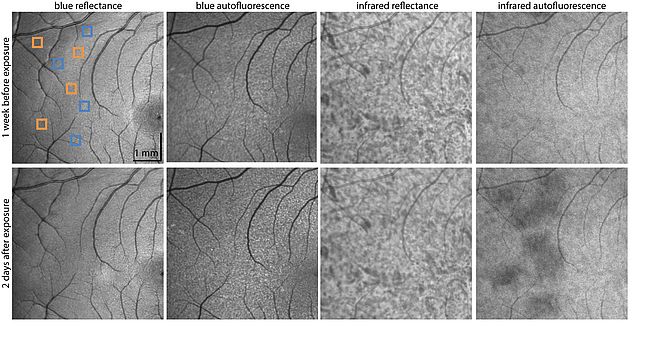
Light safety of sub-100 fs lasers (as those used for TPO) is of major concern because safety standards are not yet well-established. We examined the effects of a ~55 fs, 730 nm laser on the retina with an AOSLO. The special paradigm of dark adaptation and subsequent stimulation by the imaging laser allowed simultaneous ultrashort pulse laser exposure, photoreceptor reflectance imaging providing structural information and functional TPEF measurements. We were able to show that the necessary light exposures for TPO did not produce any detectable cellular structural or functional changes [4]. Photoreceptors and RPE appeared normal, and the time course of TPEF was maintained, suggesting an intact visual cycle. However, these exposures caused a decrease in infrared autofluorescence intensity, which is most likely related to melanin oxidation (Fig. 3) [5]. Infrared autofluorescence intensity shows full recovery and could so far not be related to any impairment of visual function [6].
Initial sign of retinal damage following intense ultrashort pulse laser exposures
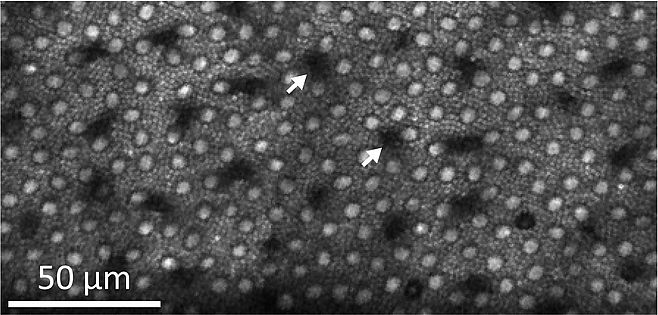
Paradoxically, intense ultrashort pulse laser exposures in the infrared cause selective damage to S cones. These cones are least sensitive to (infra)red but most sensitive to blue and UV light. Changes in outer segment morphology and a decrease in TPEF could already be observed 15 min after the damaging exposure (Fig. 4).
Over several weeks S cone outer segments degenerated until they disappeared and the surrounding photoreceptors filled in the space. Despite these substantial changes, ~12 weeks post-exposure the retina appeared morphologically and functionally intact, exhibiting a largely regular photoreceptor mosaic and typical TPEF responses to stimulation. Control experiments with temporally broadened laser pulses and theoretical calculations of photoreceptor stimulation support the hypothesis that a nonlinear effect is involved in the damage mechanism. Nevertheless, the fact that S cone-damaging exposures exceed those required for functional two-photon ophthalmoscopy 40-fold indicates that safe TPO in people is within reach.
Coming soon: Functional two-photon ophthalmoscopy in human
Exposures with lasers of pulse durations below 100 fs have never been delivered intentionally to the human retina. At the University of Rochester, we have been working with the US Food and Drug Administration’s (FDA) division for retinal imaging on a study protocol for a First in Human study. As of this year we are granted approval to initiate a safety study of functional two-photon ophthalmoscopy in human. While I am still involved in this project, I recently joined the Institute for Ophthalmic Research at the University of Tübingen where I am in the process of establishing a research group that focuses on high-resolution retinal imaging and testing.
References
- J. J. Hunter, B. Masella, A. Dubra, R. Sharma, L. Yin, W. H. Merigan, G. Palczewska, K. Palczewski, and D. R. Williams, “Images of photoreceptors in living primate eyes using adaptive optics two-photon ophthalmoscopy.,” Biomed. Opt. Express, vol. 2, no. 1, pp. 139–148, Jan. 2011.
- R. Sharma, C. Schwarz, D. R. Williams, G. Palczewska, K. Palczewski, and J. J. Hunter, “In Vivo Two-Photon Fluorescence Kinetics of Primate Rods and Cones,” Investig. Opthalmology Vis. Sci., vol. 57, no. 2, pp. 647–657, 2016.
- R. Sharma, C. Schwarz, J. J. Hunter, G. Palczewska, K. Palczewski, and D. R. Williams, “Formation and Clearance of All- Trans -Retinol in Rods Investigated in the Living Primate Eye With Two-Photon Ophthalmoscopy,” Investig. Opthalmology Vis. Sci., vol. 58, no. 1, pp. 604–613, 2017.
- C. Schwarz, R. Sharma, W. S. Fischer, M. Chung, G. Palczewska, K. Palczewski, D. R. Williams, and J. J. Hunter, “Safety assessment in macaques of light exposures for functional two-photon ophthalmoscopy in humans,” Biomed. Opt. Express, vol. 7, no. 12, p. 5148, 2016.
- C. N. Keilhauer and F. C. Delori, “Near-infrared autofluorescence imaging of the fundus: Visualization of ocular melanin,” Investig. Ophthalmol. Vis. Sci., vol. 47, no. 8, pp. 3556–3564, 2006.
- B. D. Masella, D. R. Williams, W. S. Fischer, E. A. Rossi, and J. J. Hunter, “Long-term reduction in infrared autofluorescence caused by infrared light below the maximum permissible exposure,” Investig. Ophthalmol. Vis. Sci., vol. 55, no. 6, pp. 3929–3938, 2014.

Christina Schwarz, PhD
Institute for Ophthalmic Research
University of Tübingen
Elfriede-Aulhorn Str. 7
72076 Tübingen
Germany