You are here: vision-research.eu » Vision Research » The Young Researchers View » Dominic Eberle (Q01-2015)
Cell transplantation and gene therapy approaches for the treatment of retinal degenerative disorders
 |
Photoreceptors are of prime importance for humans, since vision is one of the most important senses for us. In our daily life, where nearly every action is dependent on visual input, an impairment or a loss of eyesight leads to severe disability.
With a non-syndromic prevalence of 1:4000, retinitis pigmentosa, a collective term for a group of inherited retinal eye diseases, represents, together with age-related macula degeneration, one of the main causes for visual impairment and blindness in industrialized countries. The dominant reason for vision loss is, in both cases, the irreversible loss of photoreceptor cells located at the outer nuclear layer of the retina. To date, no effective treatment is available to preserve or regain visual function in affected patients.
Two examples of recent promising strategies for new retinal therapeutical approaches focus on one hand on the development of gene therapies, where an introduced wild-type allele compensates a mutated gene, and on the other hand on cell therapies, where stem or photoreceptor precursor cells (PPCs) are transplanted to the sub-retinal space to replace degenerated host photoreceptors.
I’ve been working the last five years on two different approaches addressing the issue of non-reversible photoreceptor cell loss due to retinal degenerative diseases. On one hand, I investigated new approaches in the field of retinal cell therapy, while on the other hand I studied a gene therapeutical approach targeting prominin-1, a gene involved in retinal degenerative disorders.
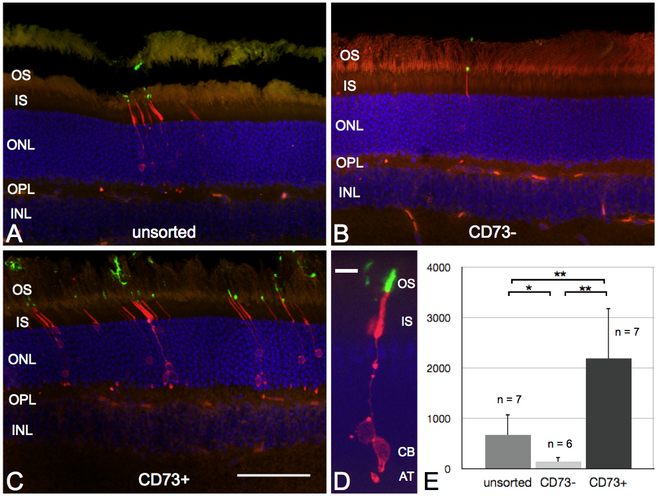
Following up on initial studies by Peter Gouras et al.1-3 already two decades ago, several recent reports demonstrated that transplantation of PPCs into the sub-retinal space of adult mice results in integration into the host outer nuclear layer (ONL) and formation of mature rod and cone photoreceptors4-7. To date, several studies use heterogeneous cell suspensions generated by dissociation of entire retinae. Consistent for all published studies, in which primary retinal cells were used for transplantation, is the relatively low number of donor-derived photoreceptors that integrated into the ONL of the host. A strategy to increase integration numbers is the enrichment of the cell suspension for the PPC fraction before transplantation. Since the purification of transplantable cells based on the expression of reporter genes will not be an approach that can be used in possible future therapeutic applications in humans, we investigated to purify donor cells by cell-type specific cell surface markers.
We were able to show this principle for ecto-5’-nucleotidase, a 70-kDa glycosyl-phosphatidylinositol (GPI)-anchored cell surface molecule, also called cluster of differentiation (CD) 73, which is highly expressed in young rod photoreceptors8-10. In our experiments, we performed magnetic-associated cell sorting (MACS) using antibodies against CD73 to enrich the population of PPCs. Upon transplantation, enriched suspensions of PPCs showed an approximately 3-fold increased integration rate into the ONL of adult mice compared to unsorted or negative sorted cell fractions. With this we could show, that by simply enriching the donor population for the right cell type, the integration rate and therefore a possible therapeutic success of such treatment strategy is significantly improved.
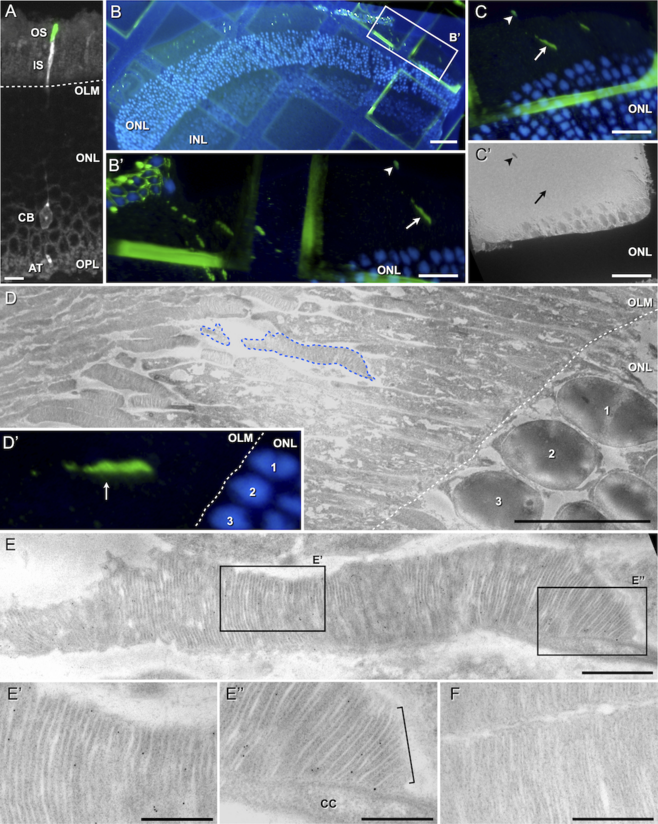
Beside this quantitative approach, I was able to show that the quality of transplanted PPCs is comparable to native photoreceptors by demonstrating, that an indispensable prerequisite of every photoreceptor cell, the outer segment (OS), is developed by transplanted PPCs after proper integration. Importantly, transplanted PPCs develop native outer segments even when not integrated into the host tissue but located at the sub-retinal space, as it is predominantly observed after transplantation into severely degenerated retinas. These results substantiate the feasibility of cell therapeutical treatment of severely degenerated retinas. Eventually, I could demonstrate, that outer segments are not formed properly by PPCs transplanted to the vitreal side of the retina. This suggests an influence of signaling molecules, presumably secreted by retinal pigment epithelial cells into the sub-retinal space, on transplanted PPC final differentiation.
The second kind of approach I was investigating was a gene therapeutical approach to cure inherited retinal degenerative diseases. Several mutations have been reported for prominin-1 to cause retinal degeneration in humans. It is a protein expressed at cell membrane evaginations in a variety of cell types. Interestingly, the prominin-1 knockout mouse is characterized exclusively by disorganized photoreceptor outer segment formation and progressive retinal degeneration. During my work I was able to successfully deliver via adeno-associated viral vector transfer a wild-type form of mouse prominin-1 into the photoreceptors of prominin-1 - deficient mice. I got divergent results showing on one hand a rescue of the thickness of the photoreceptor outer nuclear layer on a short time period (3 weeks post treatment). On the other hand long-term data (8-10 weeks post treatment) suggests histologically as well as functionally a negative effect on treated photoreceptors.
With demonstrating an increase of the integration rate of transplanted PPCs using CD73-based enrichment of the donor population for the right cell type we could set another step further towards retinal cell therapy by increasing the effectiveness of such treatment. Furthermore, the demonstration, that transplanted PPCs develop native OS, even when not integrated into the host ONL, substantiates the feasibility of such therapy and sheds light on new possibilities for the treatment of severely degenerated retinas.
References
- Gouras P, Du J, Kjeldbye H, Kwun R, Lopez R, et al. (1991) Transplanted photoreceptors identified in dystrophic mouse retina by a transgenic reporter gene. Invest Ophthalmol Vis Sci 32: 3167-3174.
- Gouras P, Du J, Kjeldbye H, Yamamoto S, Zack DJ (1992) Reconstruction of degenerate rd mouse retina by transplantation of transgenic photoreceptors. Invest Ophthalmol Vis Sci 33: 2579-2586.
- Gouras P, Du J, Kjeldbye H, Yamamoto S, Zack DJ (1994) Long-term photoreceptor transplants in dystrophic and normal mouse retina. Invest Ophthalmol Vis Sci 35: 3145-3153.
- Kwan AS, Wang S, Lund RD (1999) Photoreceptor layer reconstruction in a rodent model of retinal degeneration. Experimental neurology 159: 21-33.
- MacLaren RE, Pearson RA, MacNeil A, Douglas RH, Salt TE, et al. (2006) Retinal repair by transplantation of photoreceptor precursors. Nature 444: 203-207.
- Bartsch U, Oriyakhel W, Kenna PF, Linke S, Richard G, et al. (2008) Retinal cells integrate into the outer nuclear layer and differentiate into mature photoreceptors after subretinal transplantation into adult mice. Exp Eye Res 86: 691-700.
- Lakowski J, Baron M, Bainbridge J, Barber AC, Pearson RA, et al. (2010) Cone and rod photoreceptor transplantation in models of the childhood retinopathy Leber congenital amaurosis using flow-sorted Crx-positive donor cells. Hum Mol Genet 19: 4545-4559.
- Koso H, Minami C, Tabata Y, Inoue M, Sasaki E, et al. (2009) CD73, a novel cell surface antigen that characterizes retinal photoreceptor precursor cells. Investigative ophthalmology & visual science 50: 5411-5418.
- Lakowski J, Han YT, Pearson RA, Gonzalez-Cordero A, West EL, et al. (2011) Effective transplantation of photoreceptor precursor cells selected via cell surface antigen expression. Stem Cells 29: 1391-1404.
- Eberle D, Schubert S, Postel K, Corbeil D, Ader M (2011) Increased integration of transplanted CD73-positive photoreceptor precursors into adult mouse retina. Investigative ophthalmology & visual science 52: 6462-6471.
Dr. Dominic Eberle
Post Doctoral Researcher
Lab of Prof. Dr. Jochen Guck
Chair in Cellular Machines
Technische Universität Dresden
Biotechnology Center
Tatzberg 47/49
01307 Dresden
Germany
Contact
Technische Universität Dresden
Biotechnology Center
Phone: +49 (0)351 463 40353
dominic.eberle[at]biotec.tu-dresden.de
www.biotec.tu-dresden.de