You are here: vision-research.eu » Vision Research » The Young Researchers View » Deniz Dalkara (Q01-2014)
Deniz Dalkara and her Research Work
 |
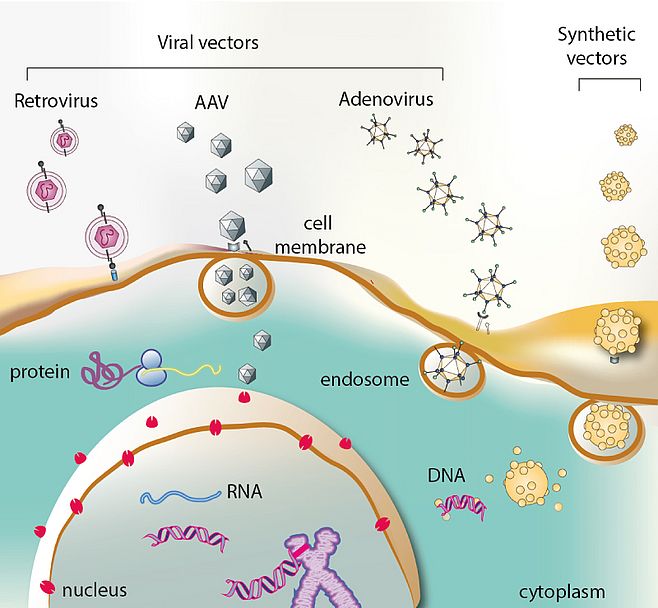
Gene therapy is the use of genetic material to treat, cure, or prevent a disease or medical condition caused by a deficient or dysfunctional protein. It involves the introduction of genetic material into a target cell. The principle of gene therapy is based on the transfer of a therapeutic gene using viral (adenoviruses, adeno-associated viruses, lentiviruses, herpes viruses) or non-viral (cationic liposomes and polymers) vectors as a result of which, the patient's cells will start to produce proteins that will correct a genetic disorder or acquired disease (Figure 01). Over the past decade significant progress has been made in our understanding of molecular and genetic mechanisms of inherited retinal degenerations. Armed with this knowledge, we have now the opportunity to develop gene-based therapies for a large number of retinal diseases. Moreover, the eye presents an ideal target for gene therapy due to its easily accessible compartmentalized structure.
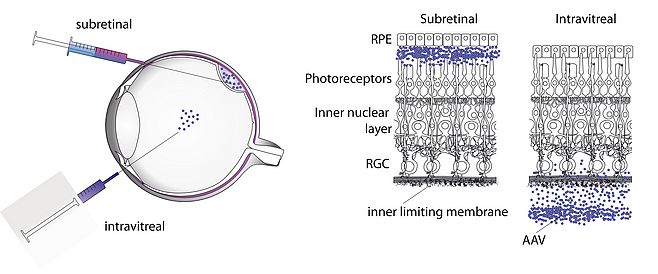
Our first objective is to develop a battery of therapeutic strategies using Adeno-associated virus (AAV) as a vector for therapeutic gene delivery to the retina. My previous work has focused on understanding the behavior of AAV after intraocular delivery and the barriers that stand in the way of efficient gene delivery using this viral particle 1,2 (Figure 2). AAV is a non-pathogenic, non-immunogenic virus that allows safe and efficient gene delivery in vivo. Using this vector we develop three distinct strategies to combat retinal degenerative disease.
Using this vector we develop three distinct strategies to combat retinal degenerative disease.
1
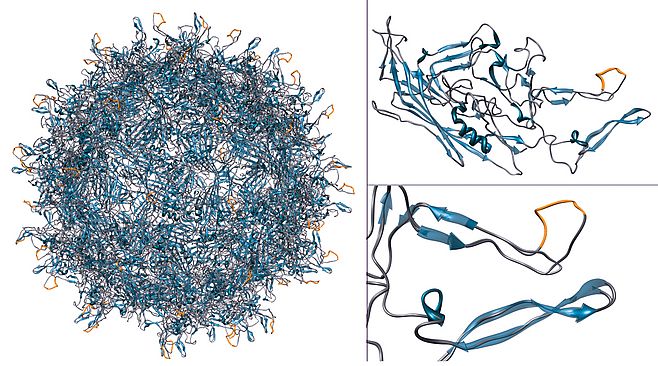
Traditional gene replacement therapy and gene knockdown therapy, targeting the photoreceptor cells of the retina are the most logical gene therapy approaches for the treatment of Retinitis Pigmentosa, and inherited monogenic diseases of the outer retina. By using rational and combinatorial strategies, we created AAVs that can transduce efficiently this therapeutically relevant cell type (Figure 03) for gene replacement or, more complex genetic strategies such as trans-splicing for mRNA repair or genome editing using TALE nucleases3. With the developments in these newly emerging technologies and ameliorations in vectors targeting the photoreceptors of the retina, we can hope to provide treatments for dominant forms of Retinitis Pigmentosa.
2
Diseases with both genetic and environmental components (such as age-related macular degeneration and glaucoma) are much more common than the monogenic diseases. Gene based therapies for these diseases, and also for diseases without any known genetic component, are often based on delivery of a neuroprotective gene. We are interested in developing this alternative gene therapeutic strategy relying on the delivery of neuro-protective or anti-apoptotic genes4,5. This mode of gene therapy equally relies on our ability to efficiently manipulate the AAV-based vectors to target various cell types in the retina and we have created a number of AAV serotypes with desirable properties in this context6,7.
3
Delivering optogenetic tools to various cell types in the retina to restore visual responses in retinas having undergone photoreceptor degeneration is an innovative approach to restore visual responses in blind retinas8. In the past years transforming non-light sensitive second and third order neurons of the retina to photoreceptive cells using optogenetic tools has been widely explored in rodent retinas9. The success of these prosthetic strategies depends on the choice of the right optogenetic tool and restricting its expression to the right cell type. To carry this strategy towards the clinic, we are exploring and optimizing expression of light-gated channels from AAV vectors in non-human primate retinas.
Gene delivery also being a research tool, we are interested in delivering genes responsible for pathological conditions to create animal models of human diseases mimicking accurately the molecular mechanisms of disease. The choice of model animals is very important to translational research as it allows pre-clinical validation of therapeutic strategies. Thanks to our engineered gene delivery vectors and knowledge on mechanisms of neurodegenerative diseases of the retina we can envision mimicking a number of pathological conditions by somatic transgenesis namely in cases where animal models mimicking human disease are not available.
Lastly, we are interested in developing synthetic vector mediated gene delivery to the retina in parallel to gene therapy using Adeno-associated viral vectors. The small size of AAV allows easy diffusion across neural tissue and leads to continuous gene expression after a single injection making it a very favorable choice for gene delivery in the neural tissue. However the big shortcoming of AAV is its limited carrying capacity. The promoter and gene of interest need to fit between the two ITRs into a 4.7 kB expression cassette. The costly nature of the clinical grade vector production is another shortcoming along with potential immune response. In view of these shortcomings we are interested in developing non-viral approaches to gene delivery using synthetic vectors10-12.
References:
- Dalkara, D. et al. Inner limiting membrane barriers to AAV-mediated retinal transduction from the vitreous. Molecular therapy 17, 2096–2102 (2009).
- Kolstad, K. D. et al. Changes in adeno-associated virus-mediated gene delivery in retinal degeneration. Human gene therapy 21, 571–578 (2010).
- Dalkara, D. et al. In vivo-directed evolution of a new adeno-associated virus for therapeutic outer retinal gene delivery from the vitreous. Science translational medicine 5, 189ra76–189ra76 (2013).
- Dalkara, D. et al. AAV Mediated GDNF Secretion From Retinal Glia Slows Down Retinal Degeneration in a Rat Model of Retinitis Pigmentosa. Molecular therapy (2011).
- Pernet, V. et al. Long-distance axonal regeneration induced by CNTF gene transfer is impaired by axonal misguidance in the injured adult optic nerve. Neurobiol. Dis. 51, 202–213 (2013).
- Koerber, J. T. et al. Molecular evolution of adeno-associated virus for enhanced glial gene delivery. Molecular therapy 17, 2088–2095 (2009).
- Klimczak, R. R., Koerber, J. T., Dalkara, D., Flannery, J. G. & Schaffer, D. V. A novel adeno-associated viral variant for efficient and selective intravitreal transduction of rat Müller cells. PloS one 4, e7467 (2009).
- Sahel, J.-A. et al. Functional rescue of cone photoreceptors in retinitis pigmentosa. Graefe's Archive for Clinical and Experimental Ophthalmology 251, 1669–1677 (2013).
- Caporale, N. et al. LiGluR restores visual responses in rodent models of inherited blindness. Molecular therapy 19, 1212–1219 (2011).
- Fraley, A. W. et al. Cationic oligonucleotide-peptide conjugates with aggregating properties enter efficiently into cells while maintaining hybridization properties and enzymatic recognition. Journal of the American Chemical Society 128, 10763–10771 (2006).
- Courtête, J. et al. Suppression of cervical carcinoma cell growth by intracytoplasmic codelivery of anti-oncoprotein E6 antibody and small interfering RNA. Molecular cancer therapeutics 6, 1728–1735 (2007).
- Dalkara, D., Zuber, G. & Behr, J.-P. Intracytoplasmic delivery of anionic proteins. Molecular therapy 9, 964–969 (2004).
Deniz Dalkara
MSc And Ph.D
E-mail:
deniz.dalkara[at]inserm.fr
Website:
www.institut-vision.org/equipe15
Institut de la Vision
INSERM UMRS 968
17 rue Moreau
75 012 PARIS
France
Phone: (33)1 53 46 25 32
Fax : (33) 1.53.46.26.01
Mobile: 06 43 60 78 24