You are here: vision-research.eu » Vision Research » The Young Researchers View » Katrin Franke (Q01-2020)
What the eye tells the brain - information processing in the retina
 |
The Research of Katrin Franke
For most animals, including humans, vision is the dominant sense and a large fraction of the brain’s computational power is devoted to processing the incoming stream of visual information. This already starts in the eye. Other than a simple camera, the retina processes the visual input by extracting separate information channels like contrast, motion or color (reviewed in (Baden et al., 2018)). Thereby, the retina determines what is forwarded to the brain and how we see the world. Since the first functional recordings of retinal neurons in the 1950s (e.g. (Kuffler, 1953)), neurophysiologists have been interested in the number of retinal output channels and in understanding how they arise within the retinal network.
These are the questions I addressed during my PhD with Prof. Thomas Euler at the Institute for Ophthalmic Research and the Center for Integrative Neuroscience at Tübingen University. Using large-scale two-photon imaging, I recorded the light-evoked activity of thousands of mouse retinal ganglion cells -- the projection neurons of the retina. In collaboration with Prof. Philipp Berens, we then clustered the cells into distinct functional groups using an unsupervised approach (Baden et al., 2016). One important result of this work was that the number of retinal output channels available to the brain is much higher than previously thought. This finding has recently been confirmed using both anatomical (Bae et al., 2018) and genetic data (Rheaume et al., 2018), demonstrating that in mice there are ~40 parallel feature channels relaying the visual information from the eye to the brain. Interestingly, a similar approach is used by state-of-the-art artificial visual systems, so-called deep neural networks (reviewed in (Kriegeskorte, 2015)), suggesting that decomposing the visual input into multiple feature channels corresponds to an efficient strategy to encode visual information.
How is this great functional diversity present at the level of the retinal ganglion cells generated within the retina? To investigate this, we next focused on the excitatory glutamatergic drive available to retinal ganglion cells (Franke et al., 2017). We found that excitatory circuits alone generate highly correlated and redundant retinal channels and that decorrelation of these channels requires a balanced interplay of excitatory and inhibitory signals. This finding highlights the importance of inhibitory circuits in generating diverse excitatory channels, as observed at the level of the retinal output.
Following a single visual feature across all retinal layers
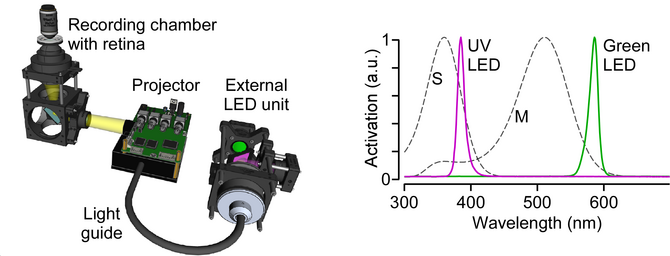
After my PhD, I accepted a position as Junior Research Group Leader at the Bernstein Center for Computational Neuroscience and Institute for Ophthalmic Research at Tübingen University. In my group, we now focus on a single visual information channel -- color -- and investigate how it arises within the retinal network. As the light sources of common monitors are optimized for the trichromatic human visual system, we – in an international collaboration with two other labs – first developed an open-source, low-cost display device (projector) with flexible light sources to allow adequate chromatic stimulation of common animal models like ultraviolet-sensitive mice and zebrafish (Franke et al., 2019).
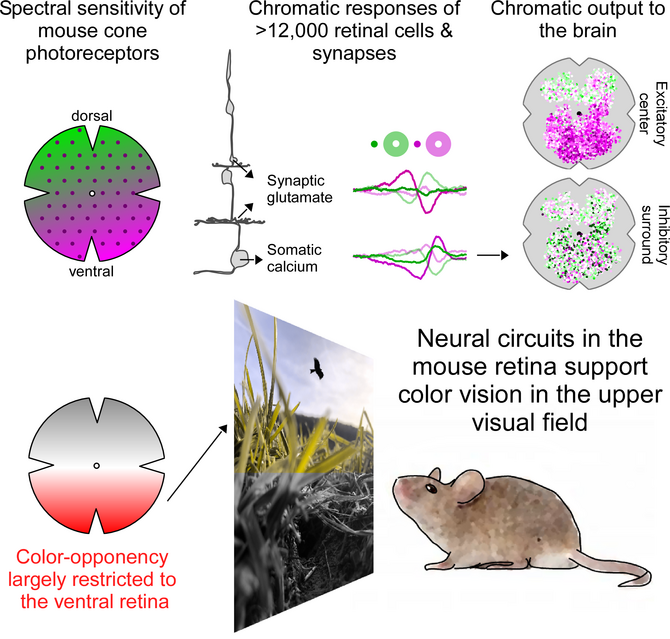
To investigate how color information is extracted by the mouse retina, we then recorded chromatic glutamate and calcium responses from >14,000 synapses and cells all the way from photoreceptors to the retinal output using two-photon imaging (Szatko et al., 2019). Surprisingly, we found that across all retinal layers color-opponent neurons -- the neural basis of color vision -- were predominantly located in the ventral retina, sampling the sky. This resonates well with recent behavioral evidence, demonstrating that color discrimination in mice might be restricted to the visual field above the horizon (Denman et al., 2018). Here, color discrimination might aid robust detection of aerial predators to ensure the animal's survival, highlighting that neural circuits in the retina are adapted to the animal´s specific visual requirements.
Coming soon - How are retinal signals processed in downstream visual brain areas?
To inform behavior, the visual information coming from the retina needs to be decoded and processed by downstream targets in the brain. In many mammals including mice, the two main routes from the eye to the brain go through the dorsal lateral geniculate nucleus (dLGN) and the superior colliculus (SC) (reviewed in (Seabrook et al., 2017)). For the dLGN, previous studies have investigated how different visual features are functionally separated in distinct regions (e.g. (Piscopo et al., 2013)) and how retinal channels converge on postsynaptic neurons (e.g. (Román Rosón et al., 2019; Rompani et al., 2017)). In contrast, much less is known about the functional organization of SC. In the future, I would like to systematically investigate how visual information coming from the retina is processed by neural circuits in the SC of mice to drive specific behaviors.
With my work, I hope to uncover fundamental principles of how the brain processes visual information encoded by the large number of retinal information channels. This will help to understand how the early visual system builds an internal representation of the external world. A thorough grasp of the first steps of vision is key when developing new treatment strategies for the diseased system. For example, identifying the best targets for restoring vision in the blind requires to know how information from the eye is processed in the brain. In addition, an increased understanding of how visual neurons robustly encode the surrounding world will provide new insights into why biological systems still outperform artificial visual systems – a prominent question in the neuroscience community.
References
- Baden T, Berens P, Franke K, Román Rosón M, Bethge M, Euler T. 2016. The functional diversity of retinal ganglion cells in the mouse. Nature 529:345–350.
- Baden T, Schubert T, Berens P, Euler T. 2018. The Functional Organization of Vertebrate Retinal Circuits for VisionOxford Research Encyclopedia of Neuroscience. Oxford University Press.
- Bae JA, Mu S, Kim JS, Turner NL, Tartavull I, Kemnitz N, Jordan CS, Norton AD, Silversmith WM, Prentki R, Sorek M, David C, Jones DL, Bland D, Sterling ALR, Park J, Briggman KL, Seung HS, Eyewirers. 2018. Digital Museum of Retinal Ganglion Cells with Dense Anatomy and Physiology. Cell 173:1293–1306.e19.
- Denman DJ, Luviano JA, Ollerenshaw DR, Cross S, Williams D, Buice MA, Olsen SR, Reid RC. 2018. Mouse color and wavelength-specific luminance contrast sensitivity are non-uniform across visual space. Elife 7. doi:10.7554/eLife.31209
- Franke K, Berens P, Schubert T, Bethge M, Euler T, Baden T. 2017. Inhibition decorrelates visual feature representations in the inner retina. Nature 542:439–444.
- Franke K, Maia Chagas A, Zhao Z, Zimmermann MJ, Bartel P, Qiu Y, Szatko KP, Baden T, Euler T. 2019. An arbitrary-spectrum spatial visual stimulator for vision research. Elife 8. doi:10.7554/eLife.48779
- Kriegeskorte N. 2015. Deep Neural Networks: A New Framework for Modeling Biological Vision and Brain Information Processing. Annu Rev Vis Sci 1:417–446.
- Kuffler SW. 1953. Discharge patterns and functional organization of mammalian retina. J Neurophysiol 16:37–68.
- Piscopo DM, El-Danaf RN, Huberman AD, Niell CM. 2013. Diverse visual features encoded in mouse lateral geniculate nucleus. J Neurosci 33:4642–4656.
- Rheaume BA, Jereen A, Bolisetty M, Sajid MS, Yang Y, Renna K, Sun L, Robson P, Trakhtenberg EF. 2018. Single cell transcriptome profiling of retinal ganglion cells identifies cellular subtypes. Nat Commun 9:2759.
- Román Rosón M, Bauer Y, Kotkat AH, Berens P, Euler T, Busse L. 2019. Mouse dLGN Receives Functional Input from a Diverse Population of Retinal Ganglion Cells with Limited Convergence. Neuron 102:462–476.e8.
- Rompani SB, Müllner FE, Wanner A, Zhang C, Roth CN, Yonehara K, Roska B. 2017. Different Modes of Visual Integration in the Lateral Geniculate Nucleus Revealed by Single-Cell-Initiated Transsynaptic Tracing. Neuron 93:767–776.e6.
- Seabrook TA, Burbridge TJ, Crair MC, Huberman AD. 2017. Architecture, Function, and Assembly of the Mouse Visual System. Annu Rev Neurosci 40:499–538.
- Szatko KP, Korympidou MM, Ran Y, Berens P, Dalkara D, Schubert T, Euler T, Franke K. 2019. Neural circuits in the mouse retina support color vision in the upper visual field. bioRxiv. doi:10.1101/745539

Dr. Kathrin Franke
Junior Research Group Leader at the Bernstein Center for Computational Neuroscience (BCCN)
Bernstein Center for Computational Neuroscience (BCCN)
Otfried-Müller-Str. 25
72076 Tübingen
Germany