You are here: vision-research.eu » Vision Research » Visionary of the Quarter » José Cunha-Vaz (Q02-2015)
Phenotypes and Biomarkers of Diabetic Retinopathy. Personalized Medicine for Diabetic Retinopathy
 |
The research topics of José Cunha-Vaz
In 1963, when initiating my stay in London, Norman Ashton challenged me to work in diabetic retinopathy (DR), a major cause of blindness, and to focus my research on the retina and retinal vascular disease, a challenge that I accepted.
At that time, the work of Majno et al. (Majno G, Palade GE, Schoefl GI. J Biophys Biochem Cytol. 1961;11:607-26) appeared in the literature, suggesting that the venous side of the capillary circulation was the most susceptible site for alterations of vascular permeability. Considering that this finding could explain the initial changes occurring in DR, we repeated their experiments in the retina and found, to our great surprise, that the retinal vessels behaved differently from other vessels in other regions of the body or even from other vessels of the eye. (Ashton N, Cunha-Vaz JG. Arch Ophthalmol. 1965; 73:211-223). These observations led us to the discovery of tight junctions between the endothelial cells of the retinal vessels, making the retinal endothelium function as an epithelial-like barrier, (Shakib M, Cunha-Vaz JG. Exp Eye Res. 1966;5:229-234) an observation that later was confirmed also to occur in the brain (Reese TS, Karnovski MJ. Journal of Cellular Biology. 1967;34:207-217). A specific blood-retinal barrier then could be demonstrated, in the rabbit, to be mainly located in the endothelial layer of the retinal vessels (Cunha-Vaz JG, Shakib M, Ashton N. Br J Ophthalmol. 1966; 50:411-453).
After establishing the morphologic basis of the blood-retinal barrier, the next challenge was to calculate its restricted permeability, and it was then that I started to work with David Maurice. We were able to measure the permeability of the retinal vessels and to identify, for the first time, the presence of an active transport system not only on the retinal pigment epithelium, but also in the retinal vessels themselves (Cunha-Vaz JG, Maurice DM. J Physiol. 1967;191:467-486).
My scientific interest remained centered on the blood-retinal barrier and DR, and when I was finally able to assemble my first team in Coimbra, we developed and tested vitreous fluorophotometry as a clinical method to quantitate the permeability of the blood-retinal barrier in patients with diabetes (Cunha-Vaz JG, Faria de Abreu JR, Campos AJ, Figo G. Br J Ophthalmol. 1975;59:649-656).
Our studies were published for the first time in 1975, in the British Journal of Ophthalmology, showing that an alteration of the blood-retinal barrier was an early alteration occurring in the retina in diabetic patients.
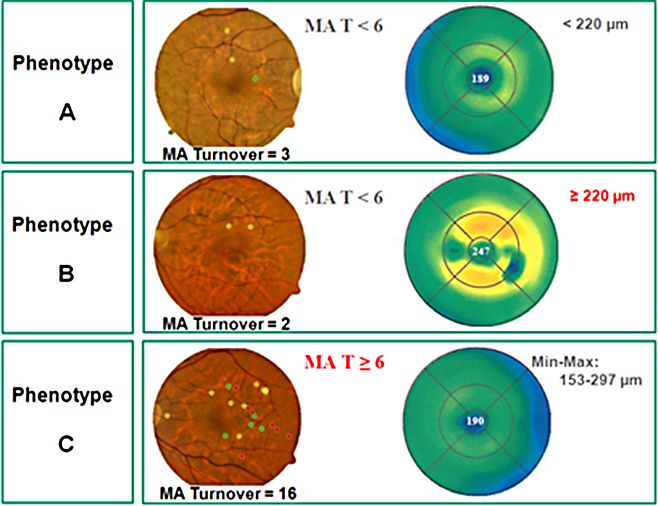
Following an invitation to Chicago, I had the unique opportunity to work with a great team at the University of Illinois Eye and Ear Infirmary and in the Lions of Illinois Eye Research Institute (1978-86). Finally, I decided to return to Portugal and Europe. Since then, I have been very much involved in bringing together European ophthalmology and fighting for the creation of an European Vision Institute along the model of the USA National Eye Institute. I was able to initiate the EuroEye Network through a research project funded by the European Union Research Programs, and this was followed more recently by the European Vision Institute Clinical Research Network, EVICR.net. Back in Europe, I continued my quest for improved understanding and management of DR. Our group, in Coimbra, introduced multimodal imaging of the macula, a methodology developed around a modified confocal scanning laser ophthalmoscope, allowing localized mapping of the alteration of the blood-retinal barrier, of particular relevance to study DR due to its initial focal nature, something that was not possible with the initial methodology used to perform vitreous fluorophotometry. (Lobo C, Bernardes R, Cunha-Vaz JG. Arch Ophthalmol. 1999;117:631-637; Bernardes R, Lobo C, Cunha-Vaz JG. Surv Ophthalmol. 2002;47:580-589). The application of multimodal mapping of the macula to diabetic patients with mild nonproliferative retinopathy revealed different phenotypes of retinopathy progression, suggesting that different patients had different types of retinal pathology, some characterized by very slow progression with minimal vascular changes occurring over time, whereas another group of patients showed mainly an alteration of the blood-retinal barrier, and, finally, a third group, showed active retinal vascular disease demonstrated by high rates of microaneurysm (MA) formation and disappearance with development of progressive capillary closure.
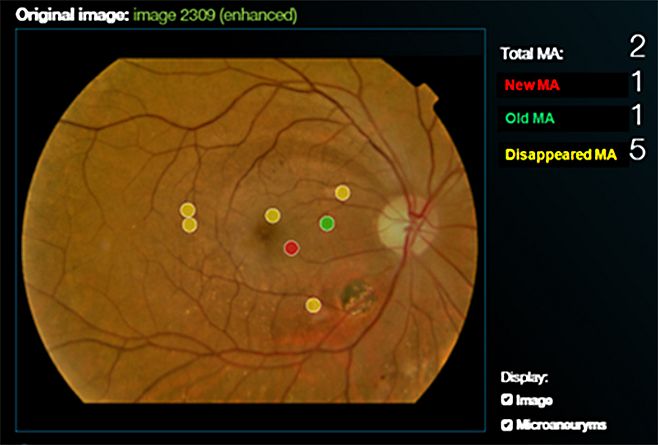
Our group patented a software to quantify MA turnover on fundus photographs, taking into account their exact, specific location in the fundus, the Retmarker. This new tool has the potential to become an extremely valuable biomarker of the overall progression of diabetic retinal vascular disease. The MA formation rate was shown to be a direct indicator of the progression of retinal vascular damage in diabetes and activity of the retinopathy (Nunes S, et al. Ophthalmologica. 2009;223:292-297; Ribeiro ML et al. Diabetes Care 2013;36:1254-1259).
In a more recent study, using the mathematical model of hierarchical cluster analysis and only noninvasive procedures (fundus photography and OCT), three different phenotypes of DR could be identified, which show different risks of progression to CSME (Nunes S et al. Invest Ophthalmol Vis Sci 2013;54(7):4595-4604).
Our research group also performed a case-control association study in type 2 diabetic patients looking for genetic biomarkers that may predict DR progression.
The results of the Multivariate Logistic Regression analysis show associations between ICAM1, PPARGC1A, and MTHFR, and the phenotypes of DR progression after adjusting for sex, age, diabetes duration, and HbA1C. The SNP ICAM1 rs1801714 was associated with phenotype B. Gene variants PPARGC1A rs10213440 and MTHFR rs1801133 were associated with phenotype C.
The multivariate analysis results also indicated an association between the gene ICAM1 and the development of CSME after adjusting for sex, age, diabetes duration, and HbA1C, suggesting that an abnormal inflammatory response may be the basis for the development of macular edema.
DR progresses over time with very little vision loss. Vision loss occurs late in the disease process and in direct association with the development of two major complications, clinically significant macular edema and proliferative retinopathy.
The challenge is to treat and stop disease progression before these complications develop. Developing precision medicine for diabetic retinopathy is my present research focus.
Prof. José Cunha-Vaz, M.D., Ph.D.
Phone: +351 239 480 136
Fax: +351 239 480 117
Email: cunhavaz[at]aibili.pt
Website: http://www.aibili.pt
Contact
AIBILI - Association for Innovation and Biomedical Research on Light and Image
Azinhaga Sta. Comba, Celas
3000-548 Coimbra
Portugal