You are here: vision-research.eu » Vision Research » Visionary of the Quarter » Phil Luthert (Q04-2020)
Understanding Disease from a Morphological Viewpoint
 |
The research work of Professor Phil Luthert
First as a neuropathologist and then as an ophthalmic pathologist I have been pre-occupied with trying to gain insights into mechanisms of disease through the use of morphological techniques. In this short article I wanted to try and show how even simple structural considerations can inform our understanding of disease and provide a framework upon which the exciting developments in molecular and cellular pathology can be integrated.
Sometimes, morphological studies are critical to inform our appreciation of the manifestations of disease. For instance, when HIV – related disease struck we were able to show that neurons were lost from the cortex and establish the need to understand the molecular basis for this. From the very simple observation that the brains of some autistic individuals were abnormally heavy we were able to show that there was distributed pathology in the cerebral hemispheres, contrary to prior dogma that the pathological substrate of autism was in the cerebellum and hindbrain.
Turning to ophthalmology perhaps the most classic inference about disease from the study of pathological eyes came from Norman Ashton who appreciated that rubeosis iridis likely arose as the result of the secretion of an hypoxia – related factor from cells in the retina, which we now know to be VEGF. Hypothesis – led science is quite rightly the cornerstone of contemporary research methodology but morphological observation provides a very valuable resource for hypothesis generation.
Age-related macular degeneration
Age-related macular degeneration (AMD) has been a long-standing interest and linking morphology to disease mechanism with Greg Hageman has been particularly rewarding. In 2001 we and others reviewed available data, which was largely morphological, and supported the notion that AMD was immune-mediated. The subsequent demonstration in 2005 that specific complement polymorphisms confer risk or protection consolidated this view but there still remains a great deal to be done to completely explain the evolution of disease and the genesis of all of the morphological hallmarks of the condition. In a separate study with Victor Chong, Greg Hageman and others we demonstrated AMD-related pathology in the elastin layer of Bruch’s membrane. It remains unclear whether this is related to neutrophil elastase or perhaps pathology linked to variants of HTRA1 but I remain convinced that protection of elastin presents an interesting therapeutic opportunity of relevance to multiple age-related disorders.
Choroidal blood flow
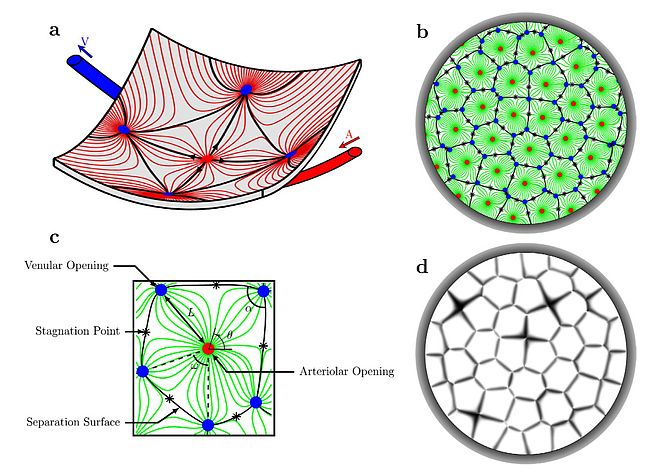
There are many physical and physiological processes that underpin how tissues work and in turn how they can fail in disease. One of the attractions of these processes is that the fundamental principles are often well known, quantitative and spatially constrained. This opens up the potential to model disease around a structural framework. Computational fluid mechanics can be used to model blood flow and with Moussa Zouache and Ian Eames I have been exploring properties of the choriocapillaris. This vascular bed, of such critical importance to photoreceptor survival and function, has an architecture totally different from the classical branching network of most microvascular beds. Modelling the patterns of flow from the arteriolar to venular insertions into the planar choriocapillaris led to some potentially counter-intuitive conclusions. Firstly, the lobular arrangement of choriocapillaris flow appears to be a function of the arrangement of arteriolar and venular insertions and the pressure and flow at each of these insertions. There is no specific anatomical organisation that defines lobular boundaries. These are defined by vascular anatomy and physiology and potentially variable. Furthermore, even though the average blood flow through the choriocapillaris is remarkably high, the modelling predicts that there are stagnation zones where flow is very low, to the extent that nutrients will have to diffuse in the plane of the choriocapillaris before exchanging with the outer retina. Given the involvement of the choroidal circulation in diabetic eye disease and AMD, understanding the impact of choroidal vasculopathy on outer retinal disease remains an important challenge.
Delivery of oxygen and glucose is likely to be limiting and much remains to be learnt about constraints arising from the delivery and clearance of other nutrients. What is emerging is that oxygen delivery has to be very tightly controlled, too much or too little can be damaging to the retina. The same may be true for glucose. A further unanswered question is how the retina tunes the choroidal circulation.
Systems Approaches to Disease
Just as ophthalmologists, pathologists get to see disease in the overall context of the tissue and organ. So the relationships between different elements can be appreciated. Particularly in age-related, complex disorders, there are co-incident changes in multiple cell types and the extracellular matrix. As multiple risk factors combine to generate disease, interactions are likely to play a central role and although classical drug – target approaches will hopefully be successful, experience from Alzheimer’s disease and related disorders gives an insight into the potential magnitude of the challenge. In recent years, some of the tools required to build systems models of disease are becoming available. The different elements of the disease can be mapped, their spatial arrangements defined, single – cell gene expression estimated and computational modelling can integrate blood flow, metabolism and detailed molecular and cellular biology.
The eye will likely be the first organ to be fully modelled in such a way. Already imaging technology is capable of delivering 3D cellular or near cellular detail. Functional imaging of blood flow and metabolism and detailed natural history studies offer the promise of deep understanding of the aging eye and the pathogenesis of important disorder such as diabetic eye disease, glaucoma, AMD, myopia etc.
There is much excitement about the potential of data science to address some of the big questions in understanding disease with the linked promise of personalised or precision medicine. There is room for multiple approaches but I would speculate that as well as data modelling, there will be a need to build bottom up, computational models of disease rooted in fundamental principles of physics, chemistry and biology that in turn will drive questions that demand the generation of new data that directly inform model building. We can look forward to paradigm – shifting approaches to chronic, age-related diseases of the eye over the next decade!
Key publications
- Everall I, Luthert PJ, Lantos PL. Neuronal loss in the frontal cortex in HIV infection. Lancet (1991) 337: 1119-1121
- Bailey A, Luthert P, Bolton P, Le Couteur A, Rutter M. Megalencephaly and autism. Lancet (1993) 341: 1225-1226
- Bailey A, Luthert P, Dean A, Harding B, Janota I, Montgomery M, Rutter M, Lantos P. A clinicopathological study of autism. Brain (1998) 121: 889-905
- Hageman Gregory S, Luthert Phil J, Chong NH Victor, Anderson Don H, Mullins Robert F. An integrated hypothesis that considers drusen as biomarkers of immune-mediated processes at the RPE-Bruch’s membrane interface in aging and age-related macular degeneration. Prog Ret Eye Res (2001) 20: 705-732.
- Chong NH, Keonin J, Luthert PJ, Frennesson CI, Weingeist DM, Wolf RL, Mullins RF, Hageman GS. Decreased thickness and integrity of the macular elastic layer of Bruch's membrane correspond to the distribution of lesions associated with age-related macular degeneration. Am J Pathol. (2005) 166: 241-51.
- Zouache MA, Eames I and Luthert PJ. Blood flow in the choriocapillaris. Journal of Fluid Mechanics. (2015) 774:37-66.
- Roberts PA, Gaffney EA, Luthert PJ, Foss AJ, Byrne HM. Retinal oxygen distribution and the role of neuroglobin. J Math Biol. (2015) Sep 14.
- Roberts PA, Gaffney EA, Luthert PJ, Foss AJ, Byrne HM. Mathematical and computational models of the retina in health, development and disease. Prog Retin Eye Res. (2016) S1350-9462(16)30010-6.
- Zouache, MA; Eames, I; Klettner, CA; Luthert, PJ. Form, shape and function: segmented blood flow in the choriocapillaris Sci Rep. (2016) 6: 35754.
- Roberts PA, Gaffney EA, Luthert PJ, Foss AJE, Byrne HM. Mathematical models of retinitis pigmentosa: The oxygen toxicity hypothesis. J Theor Biol. (2017) 21;425:53-71.
- Kiel C, Lastrucci C, Luthert PJ, Serrano L. Simple and complex retinal dystrophies are associated with profoundly different disease networks. Sci Rep. (2017) 31;7:41835.
- Roberts PA, Gaffney EA, Whiteley JP, Luthert PJ, Foss AJE, Byrne HM. Predictive Mathematical Models for the Spread and Treatment of Hyperoxia-induced Photoreceptor Degeneration in Retinitis Pigmentosa. Invest Ophthalmol Vis Sci. (2018) 59(3):1238-1249.
- Luthert PJ, Serrano L, Kiel C. Opportunities and challenges of whole-cell and tissue simulations of the outer retina in health and disease. Ann Rev Biomed Data Sci. (2018) 1: 131 – 152.
- Zouache, MA; Eames, I; Klettner, CA; Luthert, PJ. Flow and passive transport in planar multipolar flows. Journal of Fluid Mechanics /(2018) 858: 184-227.
- Kiel C, Luthert PJ. Combining Gene-Disease Associations with Single-Cell Gene Expression Data Provides Anatomy-Specific Subnetworks in Age-Related Macular Degeneration. Netw Syst Med (2020) 3: 105 – 121.
- Pool FM, Kiel C, Serrano L, Luthert PJ. Repository of proposed pathways and protein-protein interaction networks in age-related macular degeneration. NPJ Aging Mech Dis. (2020) 6: 2.
- İlhan E Acar, Laura Lores-Motta, Johanna M Colijn, Magda A Meester-Smoor, Timo Verzijden, Audrey Cougnard-Gregoire, Soufiane Ajana, Benedicte M J Merle, Anita de Breuk, Thomas J Heesterbeek, Erik van den Akker, Mohamed R Daha, Birte Claes, Daniel Pauleikhoff 7, Hans-Werner Hense, Cornelia M van Duijn, Sascha Fauser, Carel B Hoyng, Cécile Delcourt, Caroline C W Klaver , Tessel E Galesloot , Anneke I den Hollander, EYE-RISK Consortium. Integrating Metabolomics, Genomics, and Disease Pathways in Age-Related Macular Degeneration: The EYE-RISK Consortium. (2020) Ophthalmology S0161-6420(20)30561-3.

Professor Phil Luthert
BSc, FRCP, FRCPath, FRCOphth
Department of Eye Pathology
UCL Institute of Ophthalmology
London
United Kingdom
E-mail: p.luthert@ucl.ac.uk
Phone: +44207 608 6818