You are here: vision-research.eu » Vision Research » Visionary of the Quarter » Graham E Holder (Q03-2019)
From back to front and back again!
 |
A personal journey through the human visual system with clinical electrophysiology.
Clinical electrophysiology of vision plays a unique role in the investigation and management of the patient suspected of visual pathway disease by providing an objective assessment of visual pathway function. Although there have been major advances in structural imaging, both in neuroradiology and in ophthalmic imaging, electrophysiological investigation remains of paramount importance in the diagnosis and monitoring of patients with visual symptoms or of possible visual pathway dysfunction. This short account of my personal experiences also pays homage to a few individuals, as mentors or colleagues, who were instrumental to my journey.
My initial appointment, after being introduced to visual evoked potentials (VEP) by Graham Harding, was to set up evoked potentials in the regional neurosciences unit at the Brook Hospital, also doing auditory and somatosensory EPs. During this setting up period and over the following 20 years, great support was provided by a neurologist, George Harwood. The Head of Neurophysiology, Walter Kennedy, insisted on high technical standards, and it was soon shown that localisation of VEP abnormality in patients with chiasmal compression was strongly affected by stimulus and recording parameters. A number of neurologically related publications followed, including the only publication relating intraventricular metabolite levels to scalp recorded evoked potentials.
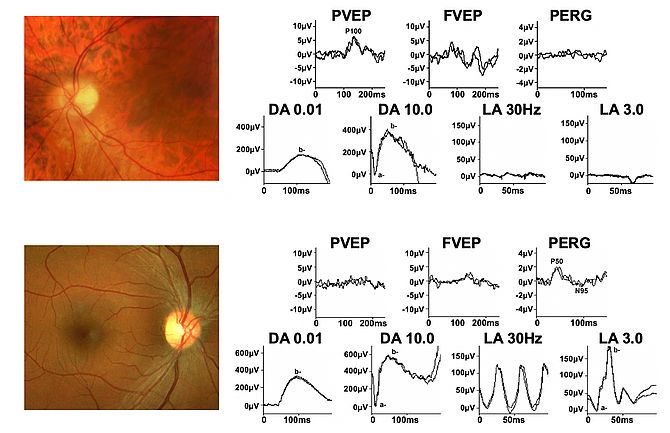
By the late 1970’s I had formed an association with John Shilling, my initial mentor in ophthalmology, and had realised that delayed VEPs, although associated with optic nerve disease were not diagnostic and could also occur in maculopathy. Thus, when Geoffrey Arden, another mentor, reported the gold foil electrode, enabling the recording of ERG signals without having to use contact lens electrodes (which affect the optics of the eye), those electrodes were rapidly adopted and a strong interest in both ERG and pattern ERG (PERG) ensued. Being in the enviable position of working with both neurological and ophthalmological patients enabled the observation that of the two main components of the PERG, now known as P50 and N95, P50 was invariably affected in macular dysfunction but could be completely spared, and selective reduction in N95 occur, in optic nerve/retinal ganglion cell dysfunction (after my initial proposal the component nomenclature was adopted by ISCEV, the International Society for Clinical Electrophysiology of Vision).
The role of the PERG in the distinction between optic neuropathy and maculopathy, often difficult clinically, and in the objective assessment of central retinal ganglion cell function, not previously possible, was established (see Fig 1). This led to the first Guidelines for PERG being published by ISCEV. Also developed was a grading system to evaluate the usefulness and clinical impact of electrophysiological investigation. The suggestion that the PERG N95 component arises in the retinal ganglion cells was later confirmed by the definitive experimental work of Laura Frishman’s group.
I was then appointed to Moorfields following Arden’s retirement and thus began a long-term collaboration with Alan Bird. They were exciting times with the importance of molecular biology coming to the fore and a large series of genotype-phenotype correlation studies were published in association with molecular biologists Shomi Bhattacharya and David Hunt, ophthalmologist Tony Moore, and psychophysicist Fred Fitzke, who at that time was developing fundus autofluorescence imaging (FAF), now an established and important part of retinal diagnostics. Andrew Webster later became an important colleague in inherited disease. Gene discovery and/or novel observations were reported in many disorders, which included enhanced S-cone syndrome (NR2E3), cone dystrophy with supernormal rod ERG (KCNV2), X-linked retinoschisis (RS1), recessive congenital stationary night blindness (TRPM1) and many others, always stressing the relationship (or lack of it) between structure and function.
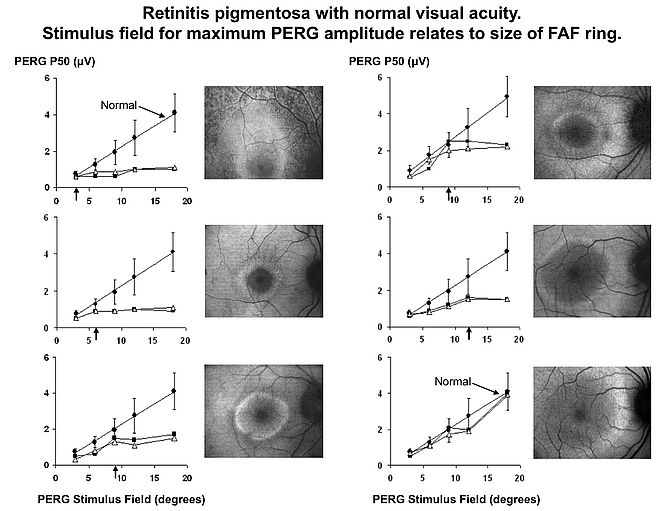
In association with Noemi Lois it was proposed that ERG could classify Stargardt disease (ABCA4 retinopathy) into 3 groups, with Group 1 having normal full field ERGs (abnormal PERG), Group 2 having abnormal full field cone ERGs, and Group 3 having additional rod ERG abnormality. Cross sectional data suggested that group 1 was associated with a better prognosis, and the prognostic value of ERG was confirmed by approximately 10 year longitudinal data from the same cohort showing that every patient with rod ERG involvement at presentation had progressive disease, both clinically and electrophysiologically, whereas only 20% of group 1 patients showed significant progression; such patients can be expected to at least maintain navigational vision. FAF imaging was an important feature of those studies, and as our experience developed it became apparent that many patients with retinitis pigmentosa have a ring of increased FAF surrounding the fovea, the significance of which was uncertain. Using pattern ERG and psychophysics we were able to establish that function within the ring is preserved, function outside the ring is lost, and there is a loss of sensitivity across the ring (see Fig 2). Subsequent developments in OCT showed the area within the ring to correspond to preservation of outer retinal structure.
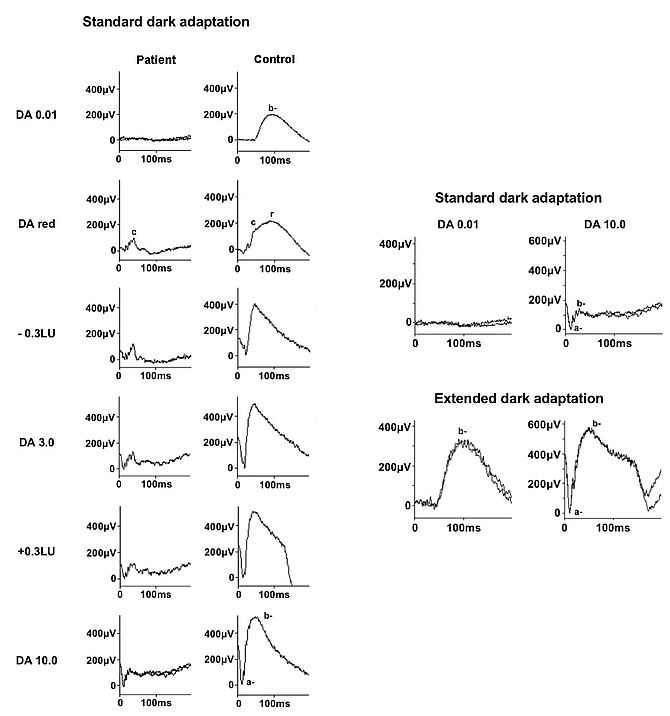
Accurate ERG interpretation requires an extensive knowledge of the origins of ERG signals and an understanding of the pathophysiology of the disorder under consideration. The demonstration of return to normal retinal function following administration of vitamin A in vitamin A deficiency, and following overnight dark adaptation in RDH5 retinopathy (fundus albipunctatus; rhodopsin levels normalise following extended DA), showed that the a “pseudo-negative ERG”, where the b-wave is of lower amplitude than the a-wave, has origins in dark adapted cones in the absence of rod function by exposing the so-called “photopic hill” phenomenon, actually a property of cones rather than the adaptive state of the retina and showed the importance of using a red stimulus under dark adaptation to give a dark-adapted cone response (see Fig 3).
The value of objective monitoring of function as an indicator of therapeutic effect was further demonstrated in birdshot retinochoroidopathy and diagnostic criteria proposed for AZOOR (acute zonal occult outer retinopathy). The phenomenon of sudden visual loss following removal of silicon oil was reported for the first time. I continued to contribute to the ISCEV Standard documents and had the privilege to Chair a committee on recommendations for visual testing in Clinical Neurophsyiology.
The next major event was the advent of treatment intervention for inherited disease. The first clinical trial in humans, for RPE65 variant Leber Congenital Amaurosis, started by Robin Ali, utilised electrophysiology both as a potential outcome measure, but also as a safety measure, showing retention of whatever function there was prior to treatment, but unfortunately there was no ERG recovery. Collaboration with Robert MacLaren on gene therapy for choroideremia utilised PERG, and in one patient during phase 1 there was loss of PERG in the untreated eye, but improvement in the treated eye. Electrophysiology was also used in the stem cell replacement therapy trial for age-related macular degeneration with Linden da Cruz. Cell replacement therapy may play a large role in the future.
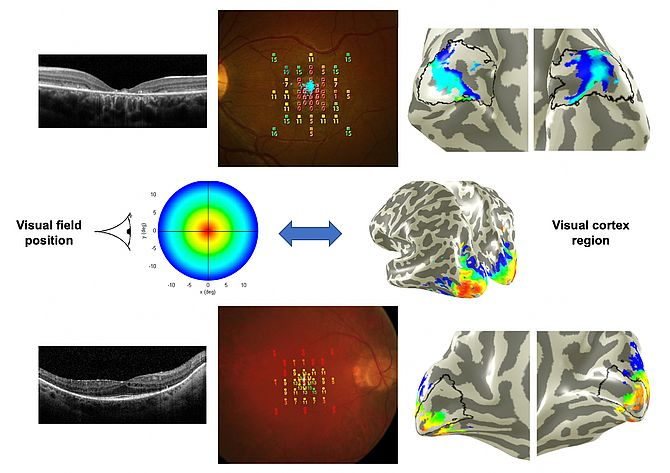
The return to “the back” of the visual system, having gone from cortical VEPs to retina, arose through collaboration with Ursula Schmidt-Erfurt and Christian Windischberger in Vienna, using f-MRI to examine cortical activation in ABCA4-related disease and RP. This is an exciting area and one which I hope will further develop.
After leaving Moorfields for a new challenge in Singapore, I have been privileged to work with Xinyi Su on the development of an artificial vitreous, which may replace silicon oil as a tamponade following retinal detachment surgery. In addition, there are many aspects of visual pathway disease in Asia which are rarely seen in Europe, just waiting for appropriate electrophysiological investigation and characterisation!
Selected publications
- Holder GE. The effects of chiasmal compression on the pattern visual evoked potential. Electroenceph clin Neurophysiol 1978; 45: 278-280.
- Holder GE, Bartlett JR, Bridges PK, Kantamaneni BD, Curzon G. Correlations between transmitter metabolite concentrations in human ventricular cerebrospinal fluid and pattern visual evoked potentials. Brain Res 1980; 188: 582-586.
- Holder GE. Pattern visual evoked potentials in patients with posteriorly situated space occupying lesions. Doc Ophthalmol 1985; 59: 121-128.
- Holder GE. The significance of abnormal pattern electroretinography in anterior visual pathway dysfunction. Br J Ophthalmol 1987; 71: 166-171. see also Editorial: Abnormal PERG Br J Ophthalmol 71: 165.
- Holder GE. Pattern electroretinography in patients with delayed pattern visual evoked potentials due to distal anterior visual pathway dysfunction. J Neurol Neurosurg Psychiat 1989; 52: 1364-1368.
- Holder GE. The incidence of abnormal pattern electroretinography in optic nerve demyelination. Electroenceph clin Neurophysiol 1991; 78: 18-26.
- Corbett MC, Shilling JS, Holder GE. The assessment of clinical investigations: the Greenwich grading system and its application to electrodiagnostic testing in ophthalmology. Eye 1995; 9 (suppl.): 59-64.
- Marmor MF, Holder GE, Porciatti V, Trick G, Zrenner E. Guidelines for pattern electroretinography. Recommendations by the International Society For Clinical Electrophysiology of Vision. Doc Ophthalmol 1996; 91: 291-298.
- Payne AM, Downes SM, Bessant DAR, Taylor R, Holder GE, Bird AC, Bhattacharya SS. Guanylate cyclase activator 1A (GUCA1A) mutation in an autosomal dominant cone dystrophy pedigree mapping to a new locus on chromosome 6p21.1. Human Molec Genet 1998; 7: 273-277.
- Votruba M, Fitzke FW, Holder GE, Carter A, Bhattacharya SS, Moore AT. Clinical features in affected individuals from 21 pedigrees with Dominant Optic Atrophy. Arch Ophthalmol 1998; 116: 351-358.
- Arden GB, Wolf J, Berninger TA, Hogg CR, Tzekov R, Holder GE. S-cone ERGs elicited by a simple technique in normals and in tritanopes. Vision Res. 1999; 39:641-650.
- Parmar DN, Sofat A, Bowman R, Bartlett JR, Holder GE. Prognostic value of the pattern electroretinogram in chiasmal compression. Br J Ophthalmol 2000; 84: 1024-1026.
- Downes SM, Holder GE, Fitzke FW, Payne AM, Warren MJ, Bhattacharya SS, Bird AC. Autosomal Dominant Cone and Cone-Rod Dystrophy with Mutations in the GUCA 1A Gene Encoding Guanylate Cyclase Activating Protein-1. Arch Ophthalmol 2001;119: 96-105.
- Holder GE. The pattern electroretinogram and an integrated approach to visual pathway diagnosis. Prog Ret Eye Res 2001; 20:531-561.
- Lois N, Holder GE, Bunce C, Fitzke FW, Bird AC. Phenotypic subtypes of Stargardt macular dystrophy - Fundus flavimaculatus. Arch Ophthalmol 2001; 119: 359-369.
- Kurz-Levin M, Halfyard AS, Bunce C, Bird AC, Holder GE. Clinical variations in assessment of Bull’s Eye Maculopathy. Arch Ophthalmol 2002; 120: 567-575.
- Dorey SE, Neveu MM, Burton L, Sloper JJ, Holder GE. The clinical features of albinism and their correlation with visual evoked potentials. Br J Ophthalmol 2003; 87: 767-772.
- Leroy BP, Hogg CR, Rath PR, McBain V, Kestelyn P, Bird AC, Holder GE. Clinical features & retinal function in patients with adult Refsum syndrome. Adv Exp Med Biol 2003; 544: 57-58.
- Robson AG, El-Amir A, Bailey C, Egan CA, Fitzke FW, Webster AR, Bird AC, Holder GE. Pattern ERG correlates of abnormal fundus autofluorescence in patients with retinitis pigmentosa and normal visual acuity. Invest Ophthalmol Vis Sci; 2003 44: 3544-3550.
- Newsom RSB, Johnston R, Sullivan PM, Aylward GW, Holder GE, Gregor ZJ. Sudden visual loss following silicon oil removal. Retina 2004; 24: 871-877.
- Francis PJ, Marinescu A, Fitzke FW, Bird AC, Holder GE. Acute zonal occult outer retinopathy (AZOOR): towards a set of diagnostic criteria. Br J Ophthalmol 2005; 89: 70-73.
- Holder GE, Robson AG, Pavesio CP, Graham EM. Electrophysiological characterisation and monitoring in the management of birdshot chorioretinopathy. Br J Ophthalmol 2005; 89: 709-718.
- Wu H, Cowing JA, Michaelides M, Wilkie SE, Jeffery G, Jenkins SA, Mester V, Bird AC, Robson AG, Holder GE, Moore AT, Hunt DM, Webster AR. Mutation of the gene KCNV2, encoding a voltage-gated potassium channel subunit, causes cone dystrophy with a supernormal rod electroretinogram in humans. Am J Hum Genet 2006; 79: 574-579.
- McBain VA, Egan CA, Pieris SJ, Supramaniam G, Webster AR, Bird AC, Holder GE. Functional observations in vitamin A deficiency: diagnosis and time-course of recovery. Eye 2007; 21: 367-376.
- Tsang SH, Vaclavik V, Bird AC, Robson AG, Holder GE. Novel Phenotypic and Genotypic Findings in X-Linked Retinoschisis (XLRS). Arch Ophthalmol 2007; 125: 259-267.
- Audo I, Michaelides M, Robson AG, Hawlina M, Vaclavik V, Sandbach JM, Neveu MM, Hogg CR, Hunt DM, Moore AT, Bird AC, Webster AR, Holder GE. Phenotypic variation in Enhanced S-cone Syndrome. Invest Ophthalmol Vis Sci 2008; 49: 2082-2093.
- Audo I, Robson AG, Holder GE, Moore AT. The negative ERG: clinical phenotypes and disease mechanisms of inner retinal dysfunction. Surv Ophthalmol 2008; 53: 16-40.
- Bainbridge JW, Smith AJ, Barker S, Robbie S, Henderson R, Balaggan K, Fitzke FW, Viswanathan A, Holder GE, Stockman A, Tyler N, Petersen-Jones S, Bhattacharya SS, Thrasher AJ, Carter BJ, Rubin GS, Moore AT, Ali RR. Effect on Visual Function in a Phase I/II Clinical Trial of Gene Therapy in Adults with Leber Congenital Amaurosis. New Eng J Med 2008; 358: 2231-2239.
- Burgess R, Millat ID, Leroy BP, Urquhart JE, Fearon IM, De Baere E, Brown PD, Robson AG, Wright GA, Kestelyn P, Holder GE, Webster AR, Manson FDC, Black GC. Biallelic mutation of BEST1 causes a distinct retinopathy in humans. Am J Hum Genet 2008; 82: 19-31.
- Mantel I, Ramchand KV, Holder GE, Ohbayashi M, Morohoshi K, Patel N, Toda M, Fitzke FW, Bird AC, Ono SJ. Macular and retinal dysfunction of unknown origin in adults with normal fundi: Evidence for an autoimmune pathophysiology. Exp Mol Pathol 2008; 84: 90-101.
- Robson AG, Michaelides M, Saihan Z, Bird AC, Webster AR, Moore AT, Fitzke FW, Holder GE. Functional characteristics of patients with retinal dystrophy that manifest abnormal parafoveal annuli of high density fundus autofluorescence; a review and update. Doc Ophthalmol. 2008; 116: 79-89.
- Li Z, Sergouniotis PI, Michaelides M, Mackay DS, Wright GA, Devery S, Moore AT, Holder GE, Robson AG, Webster AR. Recessive mutations of the gene TRPM1 abrogate ON bipolar cell function and cause complete congenital stationary night blindness in humans. Am J Hum Genet 2009; 85: 711-719.
- Holder GE, Celesia GG, Miyake Y, Tobimatsu S, Weleber RG. International Federation of Clinical Neurophysiology: Recommendations for Visual System Testing. Clin Neurophysiol 2010; 121: 1393-1409.
- Mukhopadhyay R, Holder GE, Moore AT, Webster AR. Unilateral retinitis pigmentosa occurring in an individual with a germline mutation in the RP1 gene. Arch Ophthalmol 2011; 129: 954-956.
- Sergouniotis PI, Sohn EH, Li Z, McBain VA, Wright GA, Moore AT, Robson AG, Holder GE, Webster AR. Phenotypic variability in RDH5 retinopathy (fundus albipunctatus). Ophthalmology 2011; 118: 1661-1670.
- Bach M, Brigell MG, Hawlina M, Holder GE, Johnson MA, McCulloch DL, Meigen T, Viswanathan S. ISCEV standard for clinical pattern electroretinography (PERG): 2012 update. Doc Ophthalmol 2013; 126: 1-7.
- Fujinami K, Lois N, Davidson AE, Mackay DS, Hogg CR, Stone EM, Tsunoda K, Tsubota K, Bunce C, Robson AG, Moore AT, Webster AR, Holder GE, Michaelides M. A Longitudinal Study of Stargardt Disease: Clinical and Electrophysiologic Assessment, Progression, and Genotype Correlations. Am J Ophthalmol 2013; 155: 1075-1088.e13.
- Vincent A, Robson AG, Holder GE. Pathognomonic (Diagnostic) ERGs A Review and Update. Retina 2013; 33: 5-12.
- Vincent A, Robson AG, Neveu MM, Wright GA, Moore AT, Webster AR, Holder GE. A phenotype-genotype correlation study of X-linked retinoschisis. Ophthalmology 2013; 120: 1454-1464.
- Braithwaite T, Holder GE, Lee RW, Plant GT, Tufail A. Diagnostic features of the autoimmune retinopathies. Autoimmun Rev 2014; 13: 534-538.
- Bainbridge JW, Mehat MS, Sundaram V, Robbie SJ, Barker SE, Ripamonti C, Georgiadis A, Mowat FM, Beattie SG, Gardner PJ, Feathers KL, Luong VA, Balaggan K, Viswanathan A, de Ravel TJ, Casteels I, Holder GE, Tyler N, Fitzke, FW, Weleber RG, Nardini M, Moore AT, Thompson DA, Petersen-Jones SM, Michaelides M, van der Born LI, Stockman A, Smith AJ, Rubin G, Ali RR. Long term effect of gene therapy on Leber Congenital Amaurosis. New Eng J Med 2015; 14: 372: 1887-1897.
- McCulloch DL, Marmor MF, Brigell MG, Hamilton R, Holder GE, Tzekov R, Bach M. ISCEV Standard for full-field clinical electroretinography (2015 update). Doc Ophthalmol 2015; 130:1-12.
- Edwards TL, Jolly JK, Groppe M, Barnard AR, Cottriall CL, Tolmachova T, Black GC, Webster AR, Lotery AJ, Holder GE, Xue K, Downes SM, Simunovic MP, Seabra MC, MacLaren RE. Visual acuity after retinal gene therapy for choroideremia. New Eng J Med 2016; 374: 1996-1998.
- Odom JV, Bach M, Brigell M, Holder GE, McCulloch DL, Mizota A, Tormene AP. ISCEV standard for clinical visual evoked potentials: (2016 update). Doc Ophthalmol 2016; 133: 1-9.
- Majander A, Robson AG, João C, Holder GE, Chinnery PF, Moore AT, Votruba M, Stockman A, Yu-Wai-Man P. The pattern of retinal ganglion cell dysfunction in Leber hereditary optic neuropathy. Mitochondrion 2017; 36: 138-149.
- da Cruz L, Fynes K, Georgiadis O, Kerby J, Luo YH, Ahmado A, Vernon A, Daniels JT, Nommiste B, Hasan SM, Gooljar SB, Carr AF, Vugler A, Ramsden CM, Bictash M, Fenster M, Steer J, Harbinson T, Wilbrey A, Tufail A, Feng G, Whitlock M, Robson AG, Holder GE, Sagoo MS, Loudon PT, Whiting P, Coffey PJ. Phase 1 clinical study of an embryonic stem cell-derived retinal pigment epithelium patch in age-related macular degeneration. Nature Biotechnology 2018; 36: 328-337.
- Hummer A, Ritter M, Woletz M, Ledolter AA, Tik M, Dumoulin SO, Holder GE, Schmidt-Erfurth U, Windischberger C. Artificial scotoma estimation based on population receptive field mapping. Neuroimage 2018; 169: 342-351.
- Robson AG, Nilsson J, Li S, Jalali S, Fulton AB, Tormene AP, Holder GE, Brodie SE. ISCEV guide to visual electrodiagnostic procedures. Doc Ophthalmol 2018; 136:1-26.
- Sustar M, Holder GE, Kremers J, Barnes CS, Lei B, Khan NW, Robson AG. ISCEV extended protocol for the photopic On-Off ERG. Doc Ophthalmol 2018; 136: 199-206.
- Xue K, Jolly JK, Barnard AR, Rudenko A, Salvetti AP, Patrício MI, Edwards TL, Groppe M, Orlans HO, Tolmachova T, Black GC, Webster AR, Lotery AJ, Holder GE, Downes SM, Seabra MC, MacLaren RE. Beneficial effects on vision in patients undergoing retinal gene therapy for choroideremia. Nature Medicine 2018 Oct 24(10): 1507-1512.
- Errera MH, Robson AG, Wong T, Hykin PG, Pal B, Sagoo MS, Pavesio CE, Moore AT, Webster AR, Holder GE. Unilateral pigmentary retinopathy: a retrospective case series. Acta Ophthalmol 2019; 97: e601-e617.
- Liu Z, Liow SS, Lai SL, Alli-Shaik A, Holder GE, Parikh BH, Krishnakumar S, Li Z, Tan MJ, Gunaratne J, Barathi VA, Hunziker W, Lakshminarayanan R, Tan CWT, Chee CK, Zhao P, Lingam G, Loh XJ, Su X. Retinal-detachment repair and vitreous-like-body reformation via a thermogelling polymer endotamponade. Nature Biomedical Engineering 2019 Apr 8. [Epub ahead of print]
- Ritter M, Hummer A, Ledolter A, Holder GE, Windischberger C, Schmidt-Erfurth UM. Correspondence between retinotopic cortical mapping and conventional functional and morphologic assessment of retinal disease. Br J Ophthalmol 2019; 103: 208-215.
Publication metrics (Google Scholar June 30th 2019) - https://scholar.google.co.uk/citations?user=fpTmGbUAAAAJ&hl=en
Citation Indices:
- Citations 17082
- h-index 62
- i10 index 205

Professor Graham E Holder
Hong-Leong Professor
National University of Singapore
National University Hospital
No. 1E Kent Ridge Road
National University Health System Tower Block
Department of Ophthalmology, Level 7
Singapore 119228
Tel: (+65) 6772 3975
Fax: (+65) 6777 7161
email: ophgeh[at]nus.edu.sg