You are here: vision-research.eu » Vision Research » Visionary of the Quarter » Usha Chakravarthy (Q01-2018)
The evolution of modern treatment paradigms in patients with neovascular AMD
 |
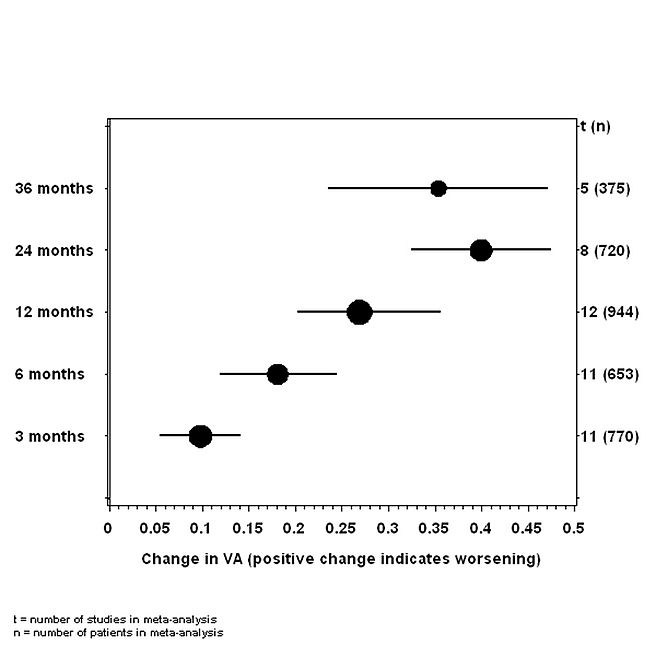
Neovascular (wet AMD) has been and continues to remain an important cause of severe visual impairment particularly in the developed world. Recognized as a disease that mainly affects the older population many centuries ago by Donders,1 during the 1980’s and 1990’s the mainstay of treatment was argon laser photocoagulation of the neovascular lesion which affects the macular fundus. The functional outcomes following thermal laser treatment were dismal with the affected eyes of most patients suffering significant vision losses within a two year period and which was confirmed by a large meta analyses (figure 1).2 Notably eyes treated with thermal laser and those in the natural history untreated arms in the many clinical studies, experienced a gradual decline of visual acuity over time with the majority of patients losing 3 to 6 lines of visual acuity within a 3 year period.
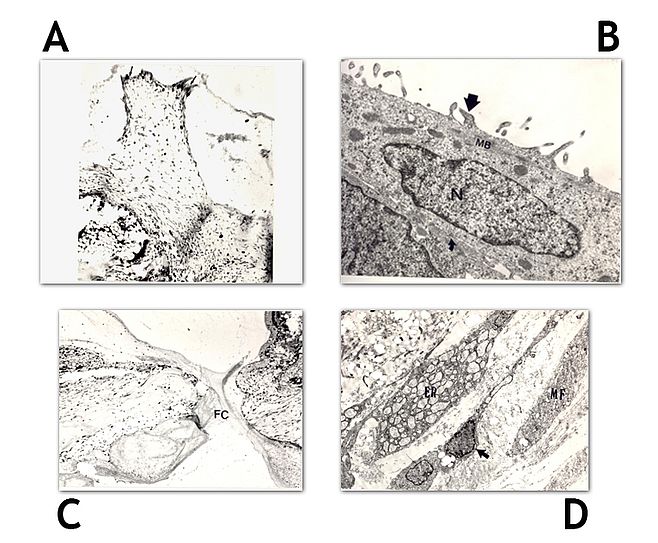
My early work on patients with established lesions of neovascular AMD showed that the main reason for vision loss occurred as a consequence of gliosis a form of retinal fibrosis that distorted and destroyed the outer retinal architecture.3 I had previously explored the use of ionizing radiation to limit intraocular fibrous proliferation (a condition akin to proliferative vitreoretinopathy) in the experimental animal model of perforating eye injury and my experiments had shown that focal radiotherapy prevented the formation of proliferating vitreoretinal membranes in (figure 2).4 Notably the evolution of granulation tissue was prevented and the ocular wound healed without forming fibrotic membranes. The inhibitory effect on fibrosis prevented onset of traction retinal detachments. In a series of dose response experiments I was able to titrate the maximum therapeutic effect with least damage to the retinal neuropile. The optimum dose appeared to lie between 12 and 15 Gy.5 Based on these experiments I designed and executed a multicenter randomized controlled clinical trial in which patients received a dose of 15 Gy of fractionated ionizing radiation ( 6 Mev photons ) to a 3 x 3 mm field of exposure at the retina. At 24 months RT exposed eyes had marginally better visual acuity compared to control eyes but the differences were not statistically significant.6 A number of secondary outcomes including near vision and contrast were found to be better 6 along with reduced fibrosis and smaller lesion sizes in the neovascular lesions in RT exposed eyes compared to eyes of control participants.7
The advent of photodynamic therapy with better functional outcomes than that obtained by RT resulted in the widespread adoption of PDT as the standard of care. However on testing PDT technology in the real world setting in a large cohort study which I led in the UK, the visual acuity effects observed in the pivotal clinical trials were not replicated and that health related patient reported quality of life outcomes indicated little or no benefit with limited cost effectiveness.,8,9
The concept of repeated intravitreal invasive administration of anti VEGF drugs was first introduced in the pegaptanib (macugen) clinical trial which utilized an aptamer designed to inhibit selective isoforms of VEGF.10 Macugen treatment resulted in superior outcomems compared to natural history and PDT. Subsequently however much greater improvements in acuity were demonstrated with a different anti VEGF ranibizumab. This antibody molecule which acted against all VEGF isoforms yielded impressive trial results and after two years of monthly treatment with ranibizumab there was average improvement in treated eyes of approximately two lines of visual acuity.11,12 This contrasted with the two line drop in acuity in untreated controls or in PDT treated eyes and a one line drop in vision in pegaptanib treated eyes. The pivotal trials of ranibizumab led to a paradigm shift in the management of wet AMD but came at a huge cost to health care providers as patients required monthly invasive administration of treatment of an expensive drug without any clear indication of when treatment may be ceased. Consequently we undertook a comparative effectiveness trial of ranibizumab versus an alternative vastly cheaper anti VEGF agent (bevacizumab) in the inhibition of VEGF in neovascular AMD (IVAN trial). The IVAN trial showed that ranibizumab and bevacizumab had equivalent effects in terms of the average improvements in distance and near acuity and reading speed.13,14 In the IVAN trial we also tested a reduced frequency of re-treatment regimen compared to monthly administration and here likewise there were no statistically significant differences in the multiple functional outcomes, though there was a marginally greater reduction in retinal thickness in the eyes of the monthly treatment group.
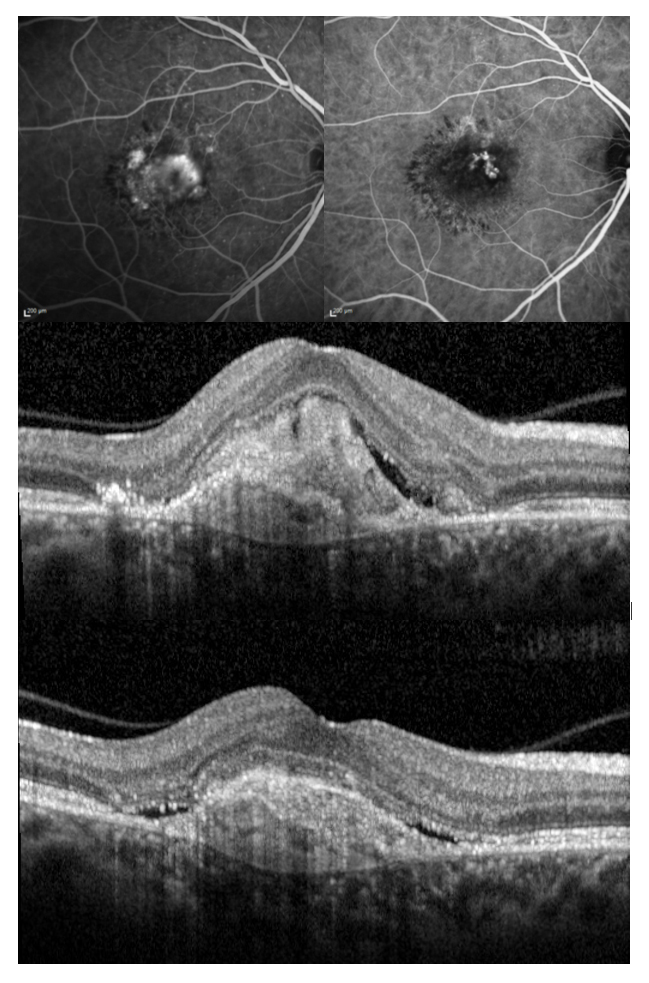
Real life UK EMR data while confirming the benefits of anti VEGF therapy compared to past treatments have revealed a worrying trend for reducing vision that over time with the average visual acuity gradually decreasing to baseline levels.14-15 These findings prompted us to take a closer look at the reasons for vision loss and our studies using high resolution multimodal imaging has confirmed a key role for fibrosis in limiting visual function. We demonstrated the relationship between fibrosis and structured defined hyperreflective material an optical coherence tomography biomarker and quantified the effects of HRM on visual acuity.15,16
An improved understanding of the evolution of fibrosis and its impact on function is a critical step in any further development of therapeutics in this field. Notably there has been a stagnation in terms of a lack of innovative newer treatment paradigms for improvements in function and or an increase in the re-treatment interval thus with reductions in the burden of treatment. The introduction of anti VEGF therapies has resulted in an enormous increase in health care resource utilization but is likely to be mitigated by the reducing levels of severe visual impairment the economic impact of which is enormous.
References
- Donders, F. (1854) Beitrage zur pathologischen Anatomie des Auges. Graefe’s Arch. Clin. Exp. Ophthalmol. 1, 106–118.
- Wong TY, Chakravarthy U Klein R, Mitchell P, Zlateva G, Buggage R, Fahrbach K, Probst C, Sledge I. The natural history and prognosis of neovascular age-related macular degeneration: a systematic review of the literature and meta-analysis. Ophthalmology. 2008 Jan;115(1):116-26.
- Hogg R, Stevenson MR, Winder J, McClure M, Chakravarthy U. Macular lesion composition and location influence visual function in AMD. Brit. J. Ophthalmol, 2003; 87:609-614
- Chakravarthy U, Biggart JH. Gardiner TA, Maguire CJF, Archer DB. A light microscopic and autoradiographic study of non-irradiated and irradiated ocular wounds. Curr. Eye. Res., 1989;8:337-348
- Chakravarthy U, Gardiner TA, Maguire CJF, Archer DB. Focal irradiation of perforating eye injuries. Curr. Eye. Res., 1989;8:1241-1250
- Hart PM, Chakravarthy U, Bird AC , Houston RF, Chakravarthy U. Subfoveal Radiotherapy Study. Visual outcomes at 12 and 24 months. Arch. Ophthalmol, 2002; 120: 1029-38
- Hart PM, Archer DB, Chakravarthy U. Asymmetry of disciform scarring in bilateral disease when one eye is treated with radiotherapy. Brit J Ophthalmol. 1995; 79:562-568
- Reeves B, Tomlin K, , Langham J, Walker J, Carpenter J, Grieve R, Harding SP, Chakravarthy U. Verteporfin Photodynamic Therapy Cohort Study. Report 2
- Grieve R, Reeves B, , Tomlin K, , Langham J, Walker J, Carpenter J, Harding SP, Chakravarthy U. Verteporfin Photodynamic Therapy Cohort Study. Report 3 Cost effectiveness and Lessons for future Evaluations
- Chakravarthy U for the VISION Study group. Year 2 efficacy results of 2 randomized controlled clinical trials of pegaptanib for neovascular age-related macular degeneration. Ophthalmology. 2006 113:.1-25
- David M. Brown, M.D., Peter K. Kaiser, M.D., Mark Michels, M.D., Gisele Soubrane, M.D., Jeffrey S. Heier, M.D., Robert Y. Kim, M.D., Judy P. Sy, Ph.D., and Susan Schneider, M.D., for the ANCHOR Study Group* Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med 2006; 355:1432-1444
- Philip J. Rosenfeld, M.D., Ph.D., David M. Brown, M.D., Jeffrey S. Heier, M.D., David S. Boyer, M.D., Peter K. Kaiser, M.D., Carol Y. Chung, Ph.D., and Robert Y. Kim, M.D., for the MARINA Study Group* Ranibizumab for neovascular age-related macular degeneration. N Engl J Med 2006; 355:1419-1431
- Chakravarthy U, Harding SP, Rogers CA, Downes SM, Lotery AJ, Wordsworth S, Reeves BC. Ranibizumab versus bevacizumab to treat neovascular age-related macular degeneration: one-year findings from the IVAN randomized trial. Ophthalmology. 2012 Jul;119(7):1399-411
- Chakravarthy U, Harding SP, Rogers CA, Downes SM, Lotery AJ, Culliford LA, Reeves BC; on behalf of the IVAN study investigators. Alternative treatments to inhibit VEGF in age-related choroidal neovascularisation: 2-year findings of the IVAN randomised controlled trial. Lancet. 2013
- Writing Committee for the UK Age-Related Macular Degeneration EMR Users Group The neovascular age-related macular degeneration database: multicenter study of 92 976 ranibizumab injections: report 1: visual acuity. Ophthalmology. 2014 May;121(5):1092-101
- Zarranz-Ventura J, Liew G, Johnston RL, Xing W, Akerele T, McKibbin M, Downey L, Natha S, Chakravarthy U, Bailey C, Khan R, Antcliff R, Armstrong S, Varma A, Kumar V, Tsaloumas M, Mandal K, Bunce C, Tufail A; United Kingdom Age-Related Macular Degeneration Electronic Medical Records Users Group. The neovascular age-related macular degeneration database: report 2: incidence, management, and visual outcomes of second treated eyes. Ophthalmology. 2014 Oct;121(10):1966-75
- Casalino G, Bandello F, Chakravarthy U. Changes in Neovascular Lesion Hyperreflectivity After Anti-VEGF Treatment in Age-Related Macular Degeneration: An Integrated Multimodal Imaging Analysis. Invest Ophthalmol Vis Sci. 2016 Jul 1;57(9):OCT288-98.
- Casalino, G, Stevenson MR, Bandello F, Chakravarthy U. Tomographic Biomarkers Predicting Progression to Fibrosis in Treated Neovascular Age-Related Macular Degeneration: A Multimodal Imaging Study
Ophthalmology Retina. Published online: October 26, 2017
Professor Usha Chakravarthy

Contact
School of Medicine, Dentistry and Biomedical Sciences
Institute for Health Sciences
Centre for Public Health
Queen’s University, Belfast
Phone: +44 (0)28 906 32636
E-mail: U.Chakravarthy[at]qub.ac.uk
Website