You are here: vision-research.eu » Vision Research » Visionary of the Quarter » Camiel J.F. Boon (Q04-2018)
Ser(i)ous research
 |
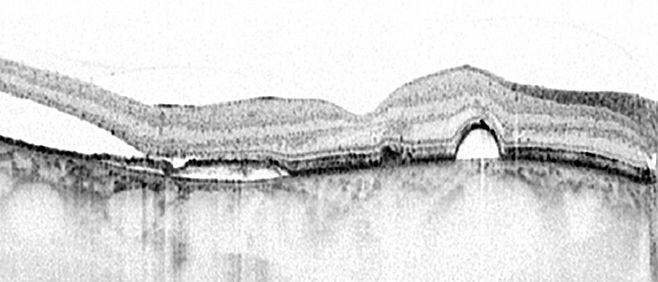
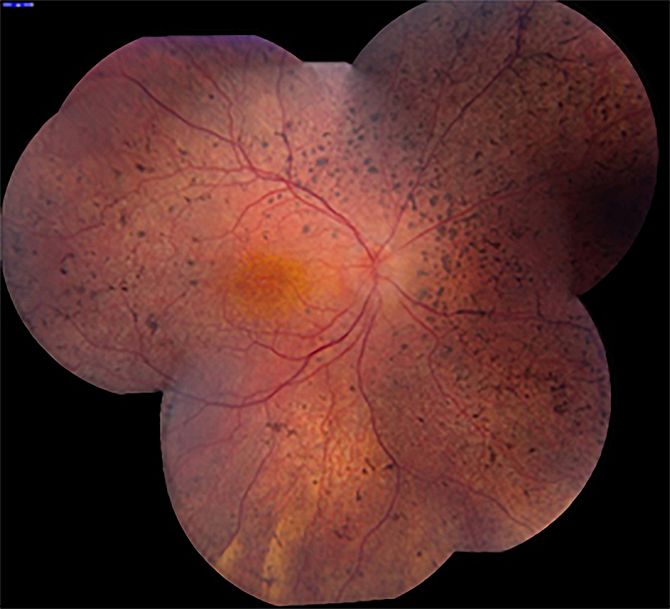
Central serous chorioretinopathy (CSC): unraveling a mysterious chorioretinal disease
In my research group, we aim to unravel CSC from pathogenesis to treatment. CSC is a relatively common disease – the fourth most common ‘wet maculopathy’ after neovascular age-related macular degeneration, diabetic macular edema and retinal vascular occlusion. CSC mainly affects men in the working age range, and is strongly associated with the use of corticosteroids. The classification of CSC is subject of debate, but many ophthalmologists make a basic distinction between acute and chronic CSC.
Genetics of CSC
Previous studies have described familial occurrence of CSC, as well as pachychoroid. In the past few years, we have identified the first genetic associations in chronic CSC. These associations include for instance genes involved in the complement system. Interestingly, several variants that were previously found to be risk-conferring in age-related macular degeneration were protective in chronic CSC, and vice versa. We also identified associations between chronic CSC and SNPs in the NR3C2 gene, which encodes the mineralocorticoid receptor. This is interesting because of the ‘stress & steroid connection’ in CSC, and also because antagonists for this receptor such as eplerenone are commonly used for the treatment of CSC.
In the near future, we aim to analyze whether the background of genetic risk factors differs between members of the spectrum of phenotypic subgroups, such as for instance typical acute CSC and severe extensive phenotypes of chronic CSC. A difference in genetic background could indicate that the different forms of CSC may also be pathophysiologically distinct, whereas shared genetic risk factors would point to pathophysiological overlap. For this purpose, we have collected the largest genetic database for CSC in the world, with more than 1500 patients with a range of CSC phenotypes. These genetic studies are performed in a multicenter collaborative setting, between LUMC, Radboud University Nijmegen Medical Center, and the Rotterdam Eye Hospital, as well as international partners. Also, we are currently performing whole exome sequencing studies in a large group of sporadic and familial CSC cases to identify potential new disease-associated genes and pathogenetic links.
CSC: Sex, Stress & Steroids
The intriguing association of CSC with male gender, steroid use, and possibly stress prompted us to dive into this pathogenetic link. In an in-depth collaborative study with the Department of Endocrinology at the LUMC, we performed a thorough analysis of the stress system (hypothalamic pituitary adrenal / HPA axis) in chronic CSC patients. After all, CSC is more common not only among steroid users but also among patients with endogenous hypercortisolism (Cushing’s syndrome), where we have shown that CSC may even be the first manifestation of Cushing’s syndrome. In chronic CSC cases, we did not identify cases of of Cushing’s syndrome, although we did find evidence of increased activity of the HPA axis.
Strikingly, this was not accompanied by increased perceived psychosocial stress or abnormal stress coping mechanisms when assessing these aspects with validated questionnaires. We are currently performing an in-depth metabolomics analysis of the hormonal and endogenous steroid system in an attempt to identify more subtle abnormalities in CSC. In addition, we have established choroidal endothelial cell model to assess relationships between corticosteroid stimulation and potential associated biological pathways that can give new insights into the pathogenesis of CSC.
CSC: A Clinical Spectrum
Thus far, the long-term prognosis of acute, focal CSC as well as chronic, extensive forms of CSC is quite unclear, and little is known about the effect of treatment. Little is known also about the risk of progression of acute CSC to chronic, extensive forms of CSC. In another large collaborative study, we have studied these aspects, and we are busy publishing our findings. It turns out that only a very small minority of acute CSC cases eventually develop toward typical chronic CSC phenotypes. This could point to essential differences between these acute, focal, self-limiting CSC phenotypes and severe progressive phenotypes. In current research projects we aim to further delineate subgroups of CSC, their clinical characteristics and course, and possible differences in treatment outcome.
Treatment of CSC: toward an evidence-based guideline
The treatment of CSC is surrounded by controversy, because of a striking lack of large prospective randomized controlled treatment trials (RCT) for CSC, and a spectrum of treatments has consequently been advocated. Because of the lack of RCTs, there is no gold standard for the treatment of CSC. Pharmaceutical industry appears to have a lack of interest to develop medication for CSC, and therefore we decided to perform our own investigator-initiated multicenter prospective randomized controlled treatment trial for chronic CSC: the PLACE trial. In this trial, we compared the two most commonly used treatments for chronic CSC: half-dose photodynamic therapy (PDT) and high-density subthreshold micropulse laser treatment (HSML). The PLACE trial is a unique collaborative European treatment trial, involving university expertise centers in the Netherlands (LUMC and Nijmegen), Cologne (Germany), Oxford (United Kingdom), and Paris (France). In this study, we were able to show that half-dose PDT was clearly superior over HSML both in terms of complete resolution of subretinal fluid on OCT, as well as certain functional parameters such as retinal sensitivity on microperimetry.
Both treatments were shown to have a very favorable safety profile. The results have been published in May 2018 in the journal Ophthalmology. In the coming years, we aim to further analyze the results of a treatment cross-over study (REPLACE), the long-term outcome and recurrence risk after treatment, as well as subgroup analyses of the PLACE trial, for instance in patients with focal versus diffuse fluid leakage. Recently, we have also started inclusion for a second RCT, the SPECTRA study (clinicaltrials.gov NCT03079141), which compares the outcome of half-dose PDT with eplerenone for the treatment of chronic CSC. The latter oral mineralocorticoid receptor antagonist medication has gained increasing popularity as first-line treatment for CSC in many countries, but prospective RCTs of its efficacy are again lacking.
The results of the SPECTRA trial are expected at the end of 2019, the same year in which the results of the VICI trial from the United Kingdom are expected, in which eplerenone is compared with sham treatment in chronic CSC. Based on these studies, we expect to be able to establish an evidence-based treatment guideline for the treatment of chronic CSC.
Monogenic retinal dystrophies: natural history studies, genotype-phenotype correlations, and endpoints for gene therapy trials
My ‘old love’ is the study of monogenetic hereditary retinal diseases, a devastating group of disorders that often result in blindness early in life, on which I did my PhD from 2005-2008 when I was still based in Nijmegen, the Netherlands. The current research field of inherited retinal diseases is very promising because of the dawn of the era of gene therapy. In this regard, the example par excellence is the successful development of clinical gene therapy for RPE65-associated retinal dystrophy and the subsequent market approval of its resulting commercial product voretigene neparvovec (Luxturna) by FDA and European Medicines Agency.
In the LUMC, we are developing gene therapy for retinal dystrophy associated with mutations in the CRB1 gene, within the group of Dr. Jan Wijnholds. For this and other genes that are potentially eligible for human gene therapy, it is essential to gain an optimal insight into the clinical course, variability, genotype-phenotype correlations, and potential endpoints for treatment. This will not only provide patients and ophthalmologists with more reliable information on the characteristics and prognosis of the disease, but may also aid in determining the optimal window of opportunity for future therapeutic interventions and best clinical parameters to assess treatment efficacy. The LUMC and Amsterdam UMC are designated Expertise Centers, resulting from a strict assessment procedure of the Dutch Federation of Universities. We aim to perform high-quality clinical and genetic studies, for instance on the CRB1 gene, the RPGR gene, the rhodopsin (RHO) gene, the CHM (choroideremia) gene, and the RS1 (X-linked juvenile retinoschisis) gene, among other genes.
In parallel, we have built a database of ‘retinas-in-a-dish’ from induced pluripotent stem cells of this patient group. In these ‘retinas-in-a-dish’, we are assessing whether the clinical phenotypes can be mimicked on a cell culture level, which would be interesting to test potential treatment options in vitro as part of a personalized medicine approach. The Delleman Database of the Amsterdam UMC – one of the largest ophthalmogenetic databases in the world, with extensive clinical and genetic data of thousands of patients – is a pivotal and unique source to achieve high-volume, high-impact studies that can give us the information that we need. Our studies are further reinforced by the inclusion of additional patients from the larger RD5000 consortium (www.rd5000.nl) on hereditary retinal dystrophies. This network of Dutch expertise centers facilitates national collaborative studies, and has resulted in numerous collaborative studies published in high-impact journals.
We also aim to establish large international studies, for instance through the European Reference Network for Rare Eye Diseases (ERN-EYE, www.ern-eye.eu), in which I am actively involved. This wonderful example of European collaboration will certainly be of great benefit to optimal care and cure for hereditary eye disease patients. However, the new EU General Data Protection Regulation rules on privacy rules do pose a challenge in facilitating national and international research initiatives.
All in all, this is an incredibly exciting time for research in the field of hereditary eye diseases. We are finally able to offer our patients some hope for treatment of their diseases, through developments such as gene therapy, stem cell therapy, and artificial vision. And I am sure that there is more ahead.

Professor C.J.F. Boon, MD, PhD, FEBO
Consultant Ophthalmologist & Clinician-Scientist, Leiden University Medical Center (LUMC) & Amsterdam University Medical Centers, the Netherlands
Leiden University Medical Center & Amsterdam University Medical Centers
The Netherlands
Email: C.J.F.Boon[at]lumc.nl