You are here: vision-research.eu » Vision Research » Visionary of the Quarter » Thomas G. Cotter (Q03-2013)
Cell Death and Survival in the Retina
 |
Background
The retina of the eye is the site of one of our five senses and is composed of an elegantly layered structure of cells (Fig 1) that are responsible for the visual process. The detection of light by photoreceptors and the subsequent down-stream neural signalling via other retinal cells ultimately leads to image formation in the brain. Unfortunately this exquisite sensory system can undergo pathological changes leading to visual impairment in a range of degenerative conditions. The loss of photoreceptors via apoptosis is a central feature of retinitis pigmentosa (RP) and age related macular degeneration (AMD). This loss in turn leads to blindness which causes significant health and associated socio-economic problems. AMD is a related and arguably more complex disease where in addition to alterations in retinal vascularization there is loss of photoreceptors.
In each of these cases the cell death occurs because of a stress stimulus that is experienced by the photoreceptor cells in the degenerating retina. Neuroprotection is one potential therapeutic strategy that can be used to protect photoreceptor cells from death and this strategy relies on the ability of growth/survival factors or other protective molecules to inhibit/retard photoreceptor apoptosis.
While we know a reasonable amount about the growth/survival factors responsible for maintaining retinal photoreceptors, we know little about their signal transduction mechanisms in the retina.
Redox survival biology
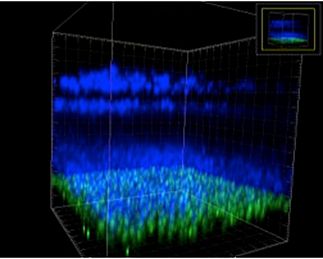
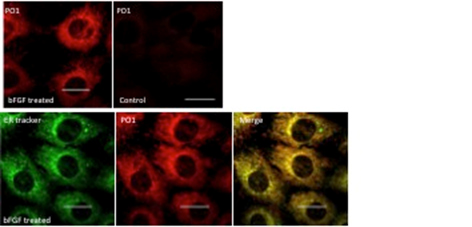
The main research focus in my laboratory trying to understand the pro-survival signalling systems that operate in photoreceptors and how they are influenced by stress and the action of their neighbouring cells. There are several strands to this research theme. In recent work we have demonstrated that FGF has a pro-survival effect on retinal photoreceptors both in vitro and in vivo. What surprised us about this work was that the generation of reactive oxygen species (ROS), hydrogen peroxide in particular, was part of the survival response. This almost counter intuitive result has drawn us into the area of redox biology and how ROS molecules can have both pro- death and pro-survival signalling actions. This effect is also seen when photoreceptors are stimulated with IGF1. In each case blocking the generation of the ROS decreased the survival response. Traditionally ROS are viewed are cell damaging molecules but an explosion of research over the last few years has clearly demonstrated that they can be produced specifically in cells in localised compartments (Fig 2) and possess signalling properties. Our current research efforts are investigating how such ROS are produced by the Nox family of enzymes and how they mediate their signalling effects in photoreceptors.
Photoreceptor neuroprotection
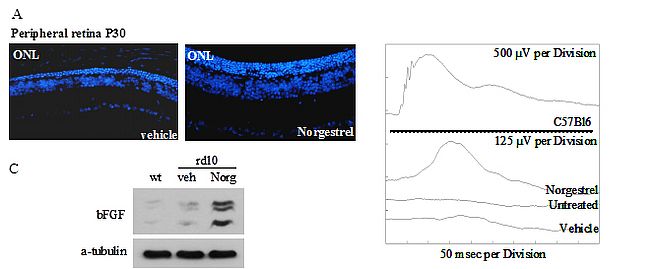
A second research theme in the laboratory is in many ways an extension of the above where we are looking for novel molecules that can act as neuroprotectants for photoreceptors and one such molecule that we discovered by screening a compound of FDA approved molecules is the synthetic progestin Norgestrel. This steroid a component of some forms of the contraceptive pill has interesting neuroprotective effects on photoreceptors in vitro and in the rd10 mouse model of RP. Our current research is trying to understand how this neuroprotectant works which interestingly seems to involve the stimulation of Muller Glial cells to produce FGF, which then acts on photoreceptors. There is also a redox component to this effect which we can see in explant cultures.
Final thoughts
While there are many potential strategies for dealing with photoreceptor death one of which is neuroprotection. Such a strategy will only succeed if we have a better understanding of how normal cell survival signalling systems work in these post mitotic cells. At the moment we know precious little about how these systems work in the retina. This in part is due to the complexity of the retina and also by the lack of suitable cell model systems. Whether neuroprotective strategies will work or not, only the future will tell, but I am an optimist in life and in research, and believe that this will be a fruitful area of eye research!
Recent publications from my laboratory
- Corcoran A, Cotter TG. Redox Regulation of Protein Kinases. FEBS J. 2013 Mar5. doi: 10.1111/febs.12224. [Epub ahead of print] PubMed PMID: 23461806.
- Woolley JF, Corcoran A, Groeger G, Landry WD, Cotter TG. Redox-Regulated Growth Factor Survival Signaling. Antioxid Redox Signal. 2013 Jan 15. [Epub aheadof print] PubMed PMID: 23198948.
- Groeger G, Doonan F, Cotter TG, Donovan M. Reactive oxygen species regulateprosurvival ERK1/2 signaling and bFGF expression in gliosis within the retina. Invest Ophthalmol Vis Sci. 2012 Sep 25;53(10):6645-54.
- Doonan F, Cotter TG. Norgestrel may be a potential therapy for retinal degenerations. Expert Opin Investig Drugs. 2012 May;21(5):579-81.
- Doonan F, Groeger G, Cotter TG. Preventing retinal apoptosis--is there a common therapeutic theme? Exp Cell Res. 2012 Jul 1;318(11):1278-84.
- Doonan F, O'Driscoll C, Kenna P, Cotter TG. Enhancing survival of photoreceptor cells in vivo using the synthetic progestin Norgestrel. J Neurochem. 2011 Sep;118(5):915-27.
- O'Driscoll C, Doonan F, Sanvicens N, Messeguer A, Cotter TG. A novel free radical scavenger rescues retinal cells in vivo. Exp Eye Res. 2011 Jul;93(1):65-74
- Farrell SM, Groeger G, Bhatt L, Finnegan S, O'Brien CJ, Cotter TG. bFGF-mediated redox activation of the PI3K/Akt pathway in retinal photoreceptor cells. Eur J Neurosci. 2011 Feb;33(4):632-41.
- Donovan M, Doonan F, Cotter TG. Differential roles of ERK1/2 and JNK inretinal development and degeneration. J Neurochem. 2011 Jan;116(1):33-42.
Professor Tom G. Cotter
UCC Biosciences Research Institute
Professor and Head of Department of Biochemistry
Contact
Department of Biochemistry
Biosciences Institute
University College Cork
Cork
Ireland
Phone: +353 21 490 1321
Fax: +353 21 490 1382
Email: t.cotter[at]ucc.ie
Website: www.ucc.ie/ucc/depts/biochemistry/staff/tcotter.html