You are here: vision-research.eu » Vision Research » Visionary of the Quarter » Francesca Cordeiro (Q01-2019)
Eyes on the brain
 |
The research work of Prof. M Francesca Cordeiro
As a clinician-scientist, my work is translational, applying molecular and biological approaches to devising methods for treatment and investigating pathogenesis of retinal neurodegenerative diseases, including glaucoma, Alzheimer’s, Parkinson's, optic neuritis and diabetes.
I have always had an interest in bio-medical research, with a lean towards clinically-relevant diagnostic and therapeutic strategies so, after ophthalmic registrar training, I was fortunate to be awarded a Wellcome Vision Research Training Fellowship which allowed me to pursue a PhD in Clinical Science with a focus on Ophthalmology on the role of the growth factor Transforming Growth Factor-ß (TGF-ß) in conjunctival scarring at University College London (UCL). This research made a meaningful impact in glaucoma surgery with the application of my preclinical studies of a novel TGF-ß2 antibody trial to clinic (until Phase III), recognized by the International Glaucoma Review Prize in 2000 to myself and supervisor Professor Sir Peng Khaw.
In 2001, I deepened my commitment to ophthalmology and neurodegeneration research by establishing a new research group at UCL supported by a Wellcome Trust University Award. The work by my team, the Glaucoma and Retinal Neurodegenerative Disease Research Group continued in the field of glaucoma, but with a new focus on the molecular and mechanical changes induced by this and other retinal neurodegenerative diseases, highlighting the eye’s role in neurodegeneration. An early highlight was the New York Academy’s Lewis Rudin Award in 2005.
My recent appointment as Chair of Imperial College Ophthalmology and its Ophthalmic Research Group (ICORG) has been key in promoting clinical research based on basic science – especially because of my combined roles of Director of the ICORG Clinical Trials Unit and Honorary Consultant Ophthalmologist at the Western Eye Hospital which offers quick and effective translation to the clinical arena, as evidenced by the Wellcome Trust-supported DARC and Anti-VEGF clinical trials.
Retinal biomarkers in neurodegenerative diseases
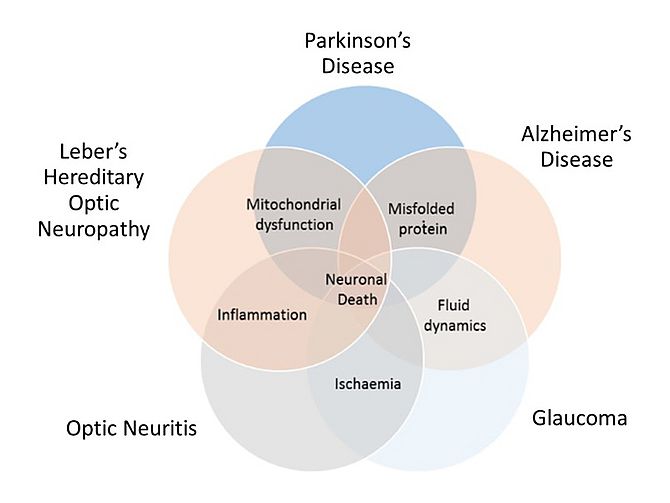
I have been a keen advocate of the retina being a ‘window’ onto the brain, given its shared anatomical and developmental origins. This importance of the retina as a model for neurodegenerative diseases is recognised more and more as evidence showing similar neuropathological processes such as inflammation, ischaemia, fluid dynamics, aggregation of misfolded proteins and mitochondrial dysfunction (oxidative stress) occur in both the retina and the brain1. These processes are, to a certain degree, common in all neurodegenerative diseases and more specific to others. As illustrated in the Venn diagram below (Figure 11), these key neuropathological mechanisms overlap with the common outcome of neuronal death. In both Parkinson’s disease (PD) and Alzheimer’s disease (AD), accumulation of misfolded proteins beta-amyloid and alpha-synuclein are thought to be central to their development2. Atypical fluid dynamics is deeply involved in both AD3 (as cerebrospinal fluid (CSF) clearance) and glaucoma4 (as intraocular pressure). Accordingly, glaucoma4 and optic neuritis5 share an ischaemic aetiology, Leber’s Hereditary optic neuropathy (LHON)6 shares inflammation with optic neuritis7 and mitochondrial dysfunction is linked to both LHON and PD8.
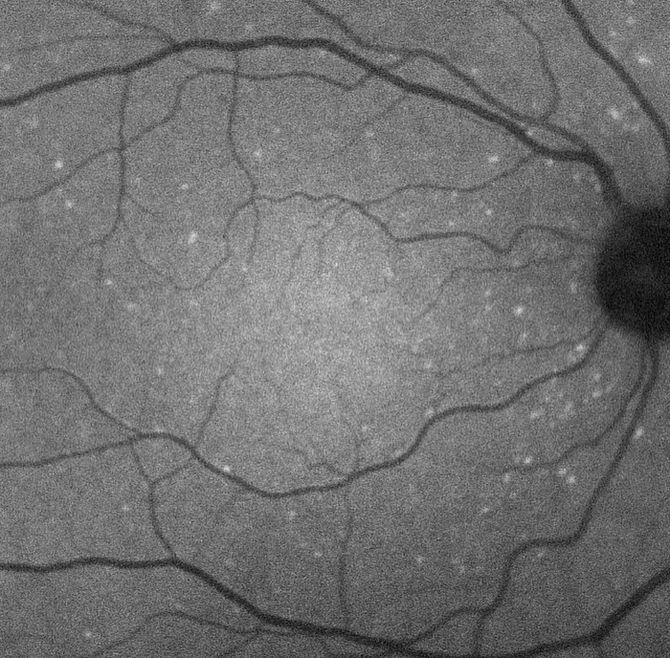
One of the exciting developments arising from the awareness of these links is a novel technique to image retinal nerve cells apoptosing in vivo. The methodology, given the acronym DARC (detection of apoptosing retinal cells) has been funded by 2 Wellcome Trust Translational Award (2009-2013) for CMC and preclinical GMP stages and Phase 1 (2013 – 2015) and 2 (2016 – 2018) clinical trials in glaucoma, macular degeneration, Down’s Syndrome, optic neuritis and healthy volunteers.
Detection of Apoptosing Retinal Cells
Apoptosis has been heralded as an early sign of pathology in many diseases, including ophthalmic and CNS neurodegeneration. Annexin A5 has been used in cell biology for years to identify apoptosis. The DARC technique enables in vivo visualization of apoptosis in the human retina at a single cell level of resolution using confocal scanning laser ophthalmoscopy following administration of intravenous fluorescently-labelled annexin. Phase 1 results were recently published and in 15 subjects (8 glaucoma and 7 healthy controls) demonstrated a significantly higher DARC count ( p = 0.0033, 2-way ANOVA) in the glaucoma group. Furthermore, it suggested the DARC count could be a surrogate of future progression in any parameter (HRT, OCT or SAP) when compared to healthy controls (Dunn’s multiple comparison test, p < 0.05). (Figure 2)
Novel drug delivery systems
My group has also been developing eye-drops and formulations to cross the blood-brain and blood-retinal barriers. Since 2016, we have been funded by a Wellcome Trust Health Innovation Award to develop anti-VEGF eye-drops. A clinical trial for this in patients with AMD is due to commence at the end of 2019. The development of novel nanotechnology drug delivery vehicles has major impact on healthcare and patient benefit, and is clearly an important area with a growing unmet need. We have also been keen to exploit the concept of targeted drug-delivery, with a view specially to developing neuroprotective regimens and gene/cell therapy.
References
- Cordeiro, M. F. Eyeing the brain. Acta Neuropathol. 132, 765–766 (2016).
- Dobson, C. M. Protein folding and misfolding. Nature 426, 884–890 (2003).
- Hart, N. J., Koronyo, Y., Black, K. L. & Koronyo-Hamaoui, M. Ocular indicators of Alzheimer’s: exploring disease in the retina. Acta Neuropathol. 132, 767–787 (2016).
- Davis, B. M., Crawley, L., Pahlitzsch, M., Javaid, F. & Cordeiro, M. F. Glaucoma: the retina and beyond. Acta Neuropathol. 132, 807–826 (2016).
- Kelley, R. E. Ischemic demyelination. Neurol. Res. 28, 334–340 (2006).
- Yu, A. K. et al. Mitochondrial complex I deficiency leads to inflammation and retinal ganglion cell death in the Ndufs4 mouse. Hum. Mol. Genet. 24, 2848–2860 (2015).
- Costello, F. Inflammatory Optic Neuropathies. Contin. Lifelong Learn. Neurol. 20, 816–837 (2014).
- Valero, T. Mitochondrial biogenesis: pharmacological approaches. Curr. Pharm. Des. 20, 5507–9 (2014).

Professor M Francesca Cordeiro, PhD MRCP FRCOphth
Professor of Glaucoma & Retinal Neurodegeneration Studies at University College London (UCL)
Chair and Professor of Ophthalmology Imperial College London
Honorary Consultant Ophthalmologist Western Eye Hospital
Director Imperial College Ophthalmology Research Group (ICORG) Clinical Trials Unit
Contact:
UCL Institute of Ophthalmology
Bath Street
London EC1V 9EL
Tel/Fax: +44(0)207 608 6938/9
Imperial College London
Imperial College Ophthalmology Research Group (ICORG) Clinical Trials Unit
Imperial College Healthcare NHS Trust, Western Eye Hospital
171 Marylebone Road
London NW1 5QH
Tel: +44(0)203 312 3206
Email: m.cordeiro[at]ucl.ac.uk
Website: http://iris.ucl.ac.uk/iris/browse/profile?upi=MFCOR19