You are here: vision-research.eu » Vision Research » Visionary of the Quarter » Andreas Reichenbach (Q03-2016)
Müller cells - essential players in retinal development and functioning
 |
The research topics of Andreas Reichenbach and his team
In the early 1980s when only a few scientists worldwide seriously considered that there is more than nerve cells in the central nervous system, I focussed my work on the radial glial (Müller) cells of the retina. Later I established what I still think was the world’s largest team devoted to Müller cell research – and, in fact, in the course of 30 years we were able to discover quite a few data about the impact of Müller cells on retina development, mature information processing, and (patho)physiology.
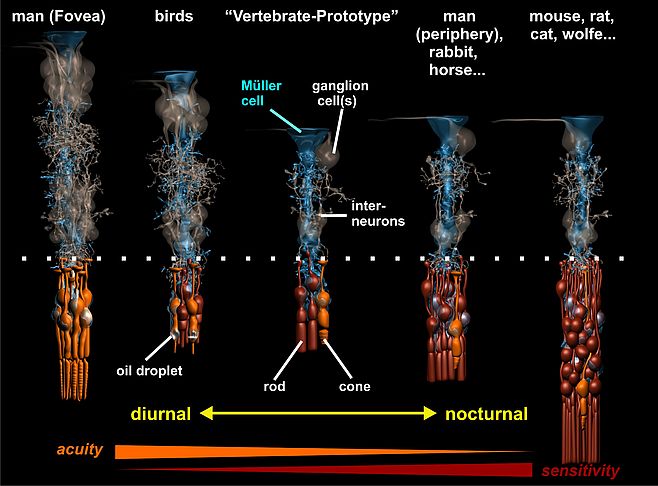
One of the most intriguing observations was the detection of the columnar organization principle of the vertebrate retina, explaining how the enormous diversity of retinal specializations (e.g., nocturnal vs. diurnal) can be generated by the variable proliferation of two types of progenitor cells and the arrangement of their neuronal descendants around a ‘core’ Müller cell (Fig. 1). Basically, we found that the retina can be envisaged as being composed of many repetitive units, each consisting of a well-defined, species (and area-) specific set of neurons. On average, in the adult mammalian retina each Müller cell is surrounded by one cone photoreceptor, three to five neurons of the inner nuclear layer, and (less than) one ganglion cell; this is what we call the ‘basic photopic set). In addition, a variable number of rods is added to this column by further proliferation of the progenitor cell(s); this ranges from less than one in the three shrew up to about 10 in humans and many other species, and more than 20 in nocturnally specialized mammals such as the cat and the mouse (Fig. 1). The idea behind this that every Müller cell represents one daughter cell of the last division of a progenitor cell, and thus, indicates the number of the primary progenitors (Reichenbach and Rbinson, 1995).
Considering the functional impact of a Müller cell for ‘its neuronal siblings’, an important step was the elucidation of the role of inwardly rectifying K+ ion channels for a diversity of neuron-supportive functions of Müller glial cells, including neurotransmitter recycling and the effective clearance of excess K+ ions and water molecules. In particular, we showed that the down-regulation of the dominant K+ conductance of the Müller cell membrane (including a re-distribution of Kir4.1 channels) in the course of retinal diseases is the key event in ‘pathological’ retinal gliosis, as it prevents the maintenance of most neuro-supportive glial functions, and allows for glial cell proliferation and scar formation. As similar functions were described for other types of glial cells, we summarized the central role of glial homeostatic mechanisms for a high signal-to-noise ratio in neuronal information processing including neuronal plasticity (Reichenbach and Pannicke, 2008).
We then applied the techniques of the just-emerging field of neuromechanics to Müller (and other glial) cells. This allowed us to show that from a biomechanical point of view, both historical views of glial cells – either as a viscous ‘glue’ or as stiff ‘support pillars’ – are wrong. Rather, glial cells are very soft, elastic ‘springs’ providing a shock-absorbing envelope of the fragile neurons, as well as a neurite growth- and plasticity-promoting substrate (Lu et al., 2006).
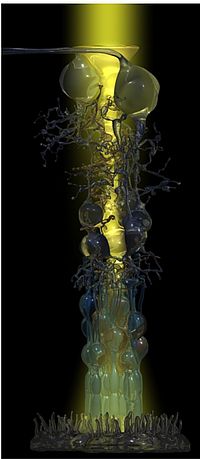
It was the collaboration with experts in biophysics, and the application of innovative techniques, which made it then possible to resolve the old enigma of high-acuity and light-sensitive vision by the inverted vertebrate retina. This retina is arranged in a manner (with the light-sensitive elements at the end of the light path through the tissue) which has been compared to “placing a thin scattering screen over the film in your camera” (Goldsmith, Q. Rev. Biol. 1990; 36:282-322). We studied the optical properties of Müller cells and of the retinal tissue which is spanned by the Müller cells throughout its thickness. In fact, we were able to show that individual Müller cells possess light-guiding properties, and that the Müller cell ‘lattice’ is comparable to an optic fiber plate, transferring the undisturbed image through the retinal layers up to the photoreceptor cells, and bypassing the light-scattering neuronal elements in the two synaptic layers (Franze et al., 2008). Noteworthy, in the retinal columns (Fig. 1) the ratio between cone photoreceptors and Müller cells is 1:1 in all mammals studied so far; thus, each cone possesses its ‘private’ light cable, delivering its particular part (‘pixel’) of the image (Fig. 2).
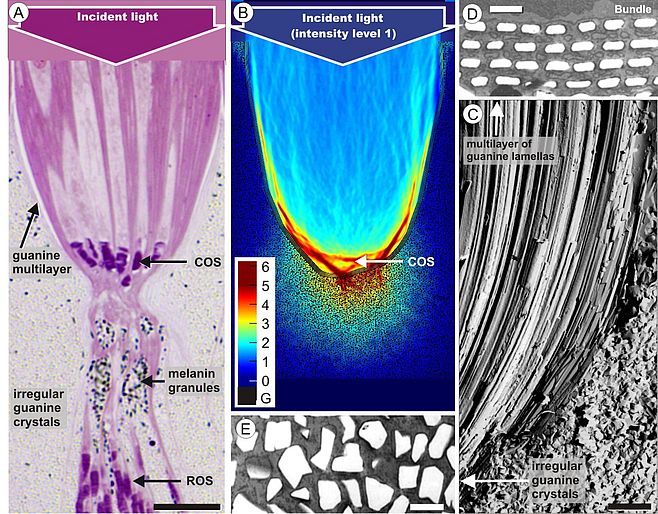
Our interest in retinal tissue optics finally prompted us to study the retina of the elephantnose fish (Gnathonemus petersii). The fish has an electric sense and is generally thought to be almost blind; it has a so-called grouped retina where large bundles of inner and outer segments of photoreceptor cells are embedded in a sandglass-shaped scabbard (Fig. 3A). The upper part of this arrangement acts like a concentrating reflector (Fig. 3B), formed by giant retinal pigment epithelial cells which contain rows of thin guanine platelets (Fig. 3C, D). Thus, in the retina of the elephantnose fish cone photoreceptors are grouped together within reflecting, photonic crystal-lined cups acting as macroreceptors, while rod photoreceptors are positioned behind these light-attenuating reflectors. This unusual arrangement matches rod and cone sensitivity for detecting color-mixed stimuli and renders the fish insensitive to spatial noise, to enable rapid flight reactions in its dim and turbid habitat (Kreysing et al., 2012). Similar specializations are found in other fishes living in turbid waters or in the deep sea (Francke et al. 2014). Thus, we have shown that two types of glial cells – Müller cells as light guiding elements in front of the photoreceptors, and pigment epithelial cells as reflectors behind them – play important roles for the vision of vertebrates.
Major publications
- Bringmann, A., Pannicke, T., Grosche, J., Francke, M., Wiedemann, P., Skatchkov, S. N., Osborne, N.N., and Reichenbach, A., Müller cells in the healthy and diseased retina. Prog. Retin. Eye Res. 25: 397–424 (2006)
- Dalloz, C., Sarig, R., Fort, P., Yaffe, D., Bordais, A., Pannicke, T., Grosche, J., Reichenbach, A., Sahel, J., Nudel, U., and Rendon, A., Targeted inactivation of dystro¬phin gene product Dp71: phenotypic impact in mouse retina. Human Mol. Genet. 12: 1543–1554 (2003)
- Finlay B, de Lima Silveira CL, Reichenbach A: Comparative Aspects of Visual System Development. In: Kremers J (ed) The Structure, Function and Evolution of the Primate Visual System. John Wiley & Sons, 2005, pp 37-72
- Francke M, Kreysing M, Mack A, Engelmann J, Karl A, Makarov F, Guck J, Kolle M, Wolburg H, Pusch R, von der Emde G, Schuster S, Wagner HJ, Reichenbach A. Grouped retinae and tapetal cups in some Teleostian fish: Occurrence, structure, and function. Prog Retin Eye Res. 2014 Jan;38:43-69.
- Franze K, Grosche J, Skatchkov SN, Schinkinger S, Foja C, Schild D, Uckermann O, Travis K, Reichenbach A, Guck J: Müller cells are living optical fibers in the vertebrate retina. PNAS 104 (2007) 8287-8292
- Germer A, Biedermann B, Schousboe A, Wolburg H, Mack A, Reichenbach A: Distribution of mitochondria within Müller cells. I. Correlation with retinal vascularization in different mammalian species. J Neurocytol 27 (1998) 329-345
- Germer A, Wolburg H, Mack A, Reichenbach A: Distribution of mitochondria within Müller cells. II. Post-natal development of the rabbit retinal periphery in vivo and in vitro: dependence on oxygen supply. J Neurocytol 27 (1998) 347-359
- Grosche A, Reichenbach A. Neuroscience. Developmental refining of neuroglial signaling? Science 339 (2013):152-153.
- Grosche, J., Matyash, V., Moller, T., Verkhratsky, A., and Reichenbach, A., and Kettenmann, H., Microdomains for neuron-glia interaction: parallel fiber signaling to Bergmann glial cells. Nat. Neurosci. 2: 139–143 (1999)
- Kreysing M, Pusch R, Haverkate D, Landsberger M, Engelmann J, Ruiter J, Mora-Ferrer C, Ulbricht E, Grosche J, Franze K, Streif S, Schumacher S, Makarov F, Kacza J, Guck J, Wolburg H, Bowmaker J, von der Emde G, Schuster S, Wagner H-J, Reichenbach A, Francke M. Photonic Crystal Light Collectors in Fish Retina Improve Vision in Turbid Water. Science 336 (2012) 1700-1703.
- Kuhrt H, Gryga M, Wolburg H, Joffe B, Grosche J, Reichenbach A, Noori HR. Postnatal mammalian retinal development: Quantitative data and general rules. Prog Retin Eye Res. 31 (2012) 605-621.
- Lu Y, Franze K, Seifert G, Steinhäuser C, Kirchhoff F, Wolburg H, Guck J, Janmey P, Käs J, Reichenbach A: Viscoelastic properties of individual glial cells and neurons in the CNS. PNAS 103 (2006) 17759-17764.
- Newman, E. A. and Reichenbach, A., The Müller cell: a functional element of the retina. Trends Neurosci. 19: 307–312 (1996)
- Reichenbach A, Bringmann A. Cell biology of the Müller cell. In S.J. Ryan (ed.) Retina, 5th Edition, Vol. 1, Elsevier-Saunders, London...2013, pp. 415-432.
- Reichenbach A, Bringmann A. Müller Cells in the Healthy and Diseased Retina. Springer, New York... 2010, pp.1-417.
- Reichenbach A, Bringmann A. New functions of Müller cells. Glia. 2013 61(5):651-678.
- Reichenbach A, Pannicke T: A new glance at glia. Science 322 (2008) 693-694.
- Reichenbach A, Robinson SR: (1995) Ependymoglia and ependymoglia-like cells. In: Ransom BR, Kettenmann H (eds): Neuroglial Cells, Oxford University Press, Oxford 1995, pp 58-84
- Reichenbach A, Robinson SR: Phylogenetic constraints on retinal organization and development: an Haeckelian perspective. Prog Retinal Res 15 (1995) 139-171
- Slezak M, Grosche A, Niemiec A, Tanimoto N, Pannicke T, Münch TA, Crocker B, Isope P, Härtig W, Beck SC, Huber G, Ferracci G, Perraut M, Reber M, Miehe M, Demais V, Lévêque C, Metzger D, Szklarczyk K, Przewlocki R, Seeliger MW, Sage-Ciocca D, Hirrlinger J, Reichenbach A, Reibel S, Pfrieger FW. Relevance of exocytotic glutamate release from retinal glia. Neuron 74 (2012) 504-516.
Prof. i. R. Dr. Andreas Reichenbach
Paul Flechsig Institute for Brain Research
Dept. Pathophysiology of Neuroglia
Leipzig University
Liebigstraße 19
D-04103 Leipzig
GERMANY
E-mail: reia[at]medizin.uni-leipzig.de