You are here: vision-research.eu » Vision Research » Visionary of the Quarter » Yvan Arsenijevic (Q02-2016)
Yvan Arsenijevic and the major topics of his research work
 |
Introduction
Human vision relies mainly on cone photoreceptors which mediate color, daylight as well as sharp and central vision. In primates, the majority of cones reside in a small pit of the central retina, the fovea. Diseases resulting in cone photoreceptor loss, such as age-related macular degeneration (AMD), Stargardt disease and other cone-rod dystrophies lead to severe visual impairment of the central vision or to legal blindness. In addition in Retinitis pigmentosa, although the disease affects primarily the rod function and survival, cones degenerate secondarily to rod loss. Numerous gene variants affect the rod or the cone functions (https://sph.uth.edu/Retnet) leading to photoreceptor death. Nonetheless, a certain degree of vision is often maintained in several of these diseases before the loss of the sensory cells.
The goals of our laboratory are to understand the mechanisms at the origin of retinal degeneration in order to identify novel therapeutic targets. Gene therapy as well as drug delivery systems are being developed with the aim of delaying photoreceptor death and thus the progression of blindness.
Molecular determinants of retinal dystrophies and identification of new therapeutic targets
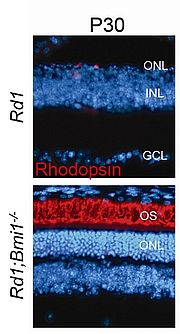
Several animal models of retinal degeneration (RD) are currently being investigated in our laboratory in order to reveal the molecular factors associated with the disease onset. Using the Rd1 mouse model (PDE6β deficiency), recent reports from our laboratory have demonstrated that the cell cycle re-entry might constitute the molecular executor that plays a central role during the photoreceptor death process (Zencak et al., 2013). Different mouse models of RD due to ciliopathy, phototransduction cascade alteration, protein aggregation, photoreceptor and retinal pigment epithelium (RPE) cell hyperactivation also lead to cell cycle re-entry following cell homeostasis instability. These results show that different forms of RD have a common cell death pathway. In the Rd1 mouse, among the cell cycle proteins/genes investigated, the Bmi1 gene plays a pivotal role in cell death execution. Inhibition of the Bmi1 gene in the Rd1 mouse leads to a photoreceptor rescue level never achieved in this model: 70% of rods and 80% of cones survive at P30, and are functional, compared to the unique row of photoreceptors out of 11 which remains at this age in the Rd1 retina. We are now attempting to elucidate the cascade of events occurring in Rd1 and Rd1;Bmi-1-/- models in order to identify novel potent biological hits. The ultimate aim is to propose innovative therapies to treat different forms of these handicapping diseases by drug or gene transfers.
Gene therapy for gene replacement strategy
Development of viral vectors as a tool for gene therapy in the eye
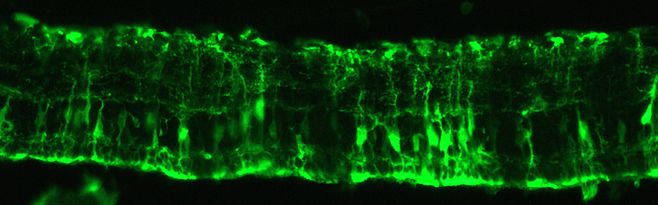
Most retinal degeneration disorders are due to photoreceptor or RPE dysfunctions, which prompted us to define appropriate tools to specifically attain these cells. We first characterized the expression of different promoters in mouse retina using lentiviral vector delivery (Kostic et al 2003). We showed that the vector is very efficient to target RPE cells and that the limited diffusion of the vectors into the retina is due to a physical barrier present between the photoreceptors and the RPE (Grüter et al 2005). Moreover we revealed that the lentiviral vector targeting also depends on the progression of the disease (Calame et al 2011). Notably, lentiviral vectors pseudotyped with the Mokola envelope efficiently reach Müller cells during the gliosis observed in the course of retinal degeneration (in the Rho-/- mouse model, Calame et al 2011). We are pursuing studies to improve lentiviral vector targeting of the retina in order to optimize gene transfer in different animal models. AAV2/8 vectors are currently used for diseases affecting the photoreceptors.
Lentiviral-mediated gene transfer for RPE65-derived Leber congenital amaurosis
Leber congenital amaurosis (LCA) is an autosomal recessive childhood-onset retinal dystrophy where RPE65 gene mutations account for 10-15% of the cases (Gu et al., 1997; Marlhens et al., 1997). We are working on a preclinical study for the treatment of LCA by lentiviral-mediated gene transfer of RPE65 in different mouse models for LCA as well as in large animals. The originality of our work is to have defined a precise therapeutic window and the effect of gene transfer on cone photoreceptors in RPE65-deficient murine models. When the gene transfer was performed during the early stages of the disease, 100% of the cones survived in the treated region and re-established their function (Bemelmans et al., 2006; Kostic). However cones were not protected when the treatment was applied in late stages of the disease (Bemelmans et al., 2006).
Considering that half of the RPE65 patients carry a missense mutation which may lead to only a partial loss of function, we evaluated the therapeutic window in a knock-in mouse model bearing the R91W mutation found in patients (Samardzija et al 2008). Interestingly in this model, not only did the gene transfer maintain the number of cones still alive at the time of injection, but it rejuvenated an additional 20% of the wild-type (WT) number of cones, that re-expressed cone markers (Kostic et al 2011). Current studies, in collaboration with Prof. F. Behar-Cohen, aim to test the safety of using such lentiviral vectors produced in conditions similar to those required for clinical applications. In parallel in collaboration with Prof. A. Kawasaki, we are evaluating how pupillary light reflex measurements could serve as an objective indicator to evaluate the retinal function in these models.
Pluripotent stem cells to investigate the physiology and pathology of human photoreceptors
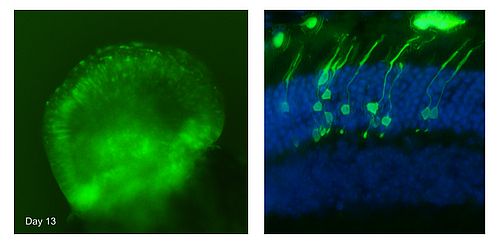
The aim of our work is to generate in vitro from human pluripotent stem cells a large number of 3D retina-like tissues (retinal organoids) bearing photoreceptors. This in vitro model should help to achieve a better comprehension of the mechanisms underlying retina development and degeneration. We have recently derived a photoreceptor-specific murine embryonic stem cell line in which a reporter gene allows to detect photoreceptors thanks to the expression of green fluorescent protein (GFP). Improving the protocol of Eiraku et al (2011), we increased the number of retina organoids and of photoreceptors (Decembrini et al 2014). Although the photoreceptors express several specific proteins, the maturation is incomplete. However, when the photoreceptors generated in culture were transplanted into adult mouse retinas, the photoreceptors fully matured and formed synapses.
Currently, our efforts are focused on the generation of human photoreceptors from induced pluripotent stem cells (iPSCs) derived from the skin and blood cells. This approach will allow us to follow the development of human retinas and photoreceptors in culture. Finally, the human retinal cultures will provide an unlimited source of photoreceptors to study retinal degeneration mechanisms and to test new therapeutic approaches.
Transgenic Pigs to translate therapy towards the clinic
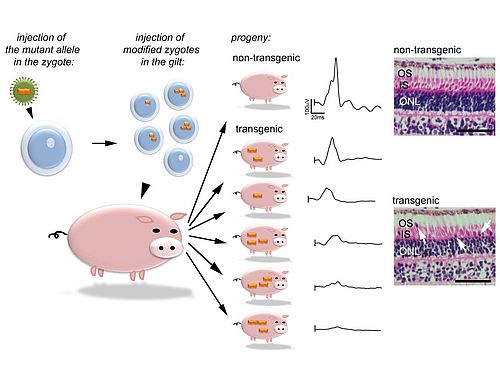
The understanding of disease mechanisms is a key factor for many biomedical researchers who dedicate their work to the development of novel therapies. Major advances have been made using animal models that reproduce human diseases; however rodents have a restricted similarity to humans concerning retinal diseases affecting cone photoreceptors. Indeed the relative distribution and organization of this type of cells in primates are particularly suited to daylight vision compared to rodents which are adapted to a nocturnal lifestyle. It would be preferable to study cone deficiency in animals that have both a cone-enriched region of the retina and an eye size that is comparable to the human. Pigs fit into this niche, and deliver a numerous progeny which is an obvious advantage for research.
In collaboration with Bruce Whitelaw (Roslin Institute, University of Edimburgh), we generated transgenic pigs bearing the GUCY2DE837D/R838S variants which are suggested to alter cone function. The transgenic pigs mimic the phenotypic range described in the human population (Kostic et al., 2013) and thus appear to be a relevant model to study cone dystrophy as well as to test preclinical approaches in a large animal model of cone dystrophy.
Key publications
- Kostic C and Arsenijevic Y 2015 Animal modeling for inherited central vision loss. J Pathol. 2015 Sep 21
- S Decembrini, U Koch, A Moulin, F Radtke, Y Arsenijevic (2014) Derivation of traceable and transplantable photoreceptors from embryonic stem cells. Stem cell Reports 22:853-65.
- C. Kostic, S.G. Lillico, S.V. Crippa,N. Grandchamp,H Pilet,S. Philippe,Z Lu, T. J. King, J. Mallet, C. Sarkis*, Y Arsenijevic*, C.B.A. Whitelaw* 2013 Rapid Cohort Generation and Analysis of Disease Spectrum of Large Animal Model of Cone Dystrophy. PLoS One Aug 19;8(8):e71363 *corresponding authors
- M Cachafeiro, A-P Bemelmans, M Samardzija, T Afanasieva, J-A Pournaras, C Grimm, C Kostic, S Philippe, A Wenzel, Y Arsenijevic. 2013 Hyperactivation of retina by light in mice leads to photoreceptors cell death mediated by VEGF and retinal pigment epithelium permeability Cell Death & Dis Aug 29;4:e781
- D Zencak, K Schouwey, D Chen, P Ekström, E Tanger, R Bremner, M van Lohuizen, Arsenijevic Y (2013) Retinal degeneration depends on Bmi1 function and re-activation of cell cycle proteins. Proc. Natl. Acad. Sci USA 110, E593-601.
- M. Calame, M. Cachafeiro, S. Philippe, M. Tekaya, D. Wanner, C. Sarkis, C. Kostic, and Y. Arsenijevic (2011) Retinal degeneration progression changes lentiviral vector cell targeting in the retina, PLoS One 2011;6(8):e23782.
- Kostic C, Crippa SV, Pignat V, Bemelmans AP, Samardzija M, Grimm C, Wenzel A, Arsenijevic Y.(2011). Gene therapy regenerates protein expression in cone photoreceptors in rpe65 mice. PLoS One. Feb 6(2):e16588.
- Langmann T, Di Gioia SA, Rau I, Stöhr H, Maksimovic NS, Corbo JC, Renner AB, Zrenner E, Kumaramanickavel G, Karlstetter M, Arsenijevic Y, Weber BH, Gal A, Rivolta C. (2010) Nonsense mutations in FAM161A cause RP28-associated recessive retinitis pigmentosa Amer. J. of Human Genetics, 87, 376-81, Aug 12. [Epub ahead of print]
- M. Cachafeiro, A.-P. Bemelmans, K. Canola, V. Pignat, S.V. Crippa, C. Kostic, and Y. Arsenijevic (2010). Remaining rod activity mediates visual behaviour in adult Rpe65-/- mice. Invest Ophthalmol Vis Sci 51, 6835-42, Aug 11. [Epub ahead of print].
- M Djojosubroto, F Bollotte, P Wirapati, F Radtke, I Stamenkovic, Y Arsenijevic (2009) Chromosomal number aberrations and transformation in adult, but not in neonatal, mouse retinal stem cells in vitro Invest Ophthalmol Vis Sci 50, 5975-87.
- A-P Bemelmans, C Kostic, SV Crippa, WW Hauswirth, J Lem, MW Seeliger, A Wenzel and Y Arsenijevic (2006). Lentiviral-mediated transfer of the Rpe65 cDNA rescues both survival and function of cone photoreceptors in a mouse model of Leber Congenital Amaurosis. PLoS Medicine Oct 10;3(10): e347
- Zencak D, Lingbeek M, Kostic C, Tekaya M, Tanger E, Hornfeld D, Jaquet M, Munier F, Schorderet D, van Lohuizen M, Arsenijevic Y. (2005) Bmi1 loss produces an increase in astroglial cells and a decrease in neural stem/progenitor cell population and proliferation. J. Neurosci. 25:5774-5783.
- Coles BL, Angenieux B, Inoue T, Del Rio-Tsonis K, Spence JR, McInnes RR, Arsenijevic** Y, van der Kooy** D. (2004) Facile Isolation and the Characterization of Human Retinal Stem Cells. Proc. Natl. Acad. Sci. U.S.A. 101(44):15772-7. Epub 2004 Oct25. **corresponding authors
Prof. Yvan Arsenijevic, PhD
Head of the Unit of Retinal Degeneration and Regeneration
Service d'Ophtalmologie
Université de Lausanne
Fondation Asile des aveugles
Hôpital Ophtalmique Jules-Gonin
15, av. de France
1004 Lausanne
Switzerland
Contact
Fondation Asile des aveugles
Hôpital Ophtalmique Jules-Gonin
Phone: +4121 626 82 60
Website: www.asile-aveugles.ch
E-mail: yvan.arsenijevic[at]fa2.ch